Abstract
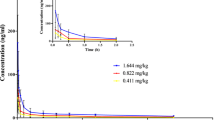
The pharmacokinetics of free and different liposomal formulations of hexadecylphosphocholine (HPC) was investigated in tumor-bearing (human mammary tumor MaTu) and tumor-free mice after intravenous and intraperitoneal administration. The levels of HPC were evaluated at different times in serum, normal tissues, and tumor. The purpose was to test the hypothesis that the enhanced therapeutic efficacy of sterically stabilized HPC liposomes in comparison to conventional vesicles and free HPC is due to its pharmacokinetics. Conventional non-compartmental pharmacokinetic analysis and an elaborate three- and four-compartmental model were used for explaining the experimental data. The serum levels of HPC obtained with sterically stabilized liposomes were only consistently higher in comparison to conventional vesicles and free HPC in the first 4 h. In the xenografted MaTu carcinoma, the differences of the HPC content between the different groups are unexpectedly low and do not reflect the high therapeutic activity [5] of sterically stabilized HPC liposomes.
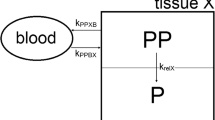
Detailed analysis shows that the liposomally encapsulated drug displays a modified pharmacokinetic behavior, which may also involve lymphatic absorption of the liposomal drug.
Similar content being viewed by others
References
Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Mattay K, Huang SK, Woodle MC, Lasic DD, Redemann C, Martin FJ: Sterically stabilized liposomes: improvements in pharmacokinetics and anti-tumor therapeutic efficacy. Proc Natl Acad Sci USA 88: 11460–11464, 1991
Arndt D, Zeisig R, Eue I, Fichtner I: Alkylphosphocholines and alkylphosphocholine liposomes. J Liposome Res 5: 91–98, 1995
Zeisig R, Eue I, Kosch M, Fichtner I, Arndt D: Preparation and properties of sterically stabilized hexadecylphosphocholine (Miltefosine)-liposomes and influence of this modification on macrophage activation. Biochim Biophys Acta 1285: 237–245, 1996
Zeisig R, Shimada K, Hirota S, Arndt D: Effect of sterical stabilization on macrophage uptake in vitro and on thickness of the fixed aqueous layer of liposomes made from alkylphosphocholines. Biochim Biophys Acta 1285: 237–245, 1996
Arndt D, Zeisig R, Eue I, Steinberg B, Fichtner I: Antineoplastic activity of sterically stabilized alkylphosphocholine liposomes in human breast carcinomas. Breast Cancer Res Treat 43: 237–246, 1997
Uziely B, Jeffers S, Isacson R, Kutsch K, Wei-Tsao D, Yeoshua Z, Libson E, Muggia F, Gabizon A: Liposomal doxorubicin: antitumor activity and unique toxicities during two complementary phase I studies J Clin Oncol 13: 1777–1785, 1995
Ranson M, Carmichael J, OByrne K, Stewart S, Smith D, Howell A: Treatment of advanced breast cancer with sterically stabilized liposomal doxorubicin: results of a multicenter phase II trial. J Clin Oncol 15: 3185–3191, 1997
Foster DM, Boston RC, Jacquez JA, Zech LA: Resource facility in kinetic analysis: modeling using the SAAM computer programs. Health Physics 57: 457–466, 1989
Rusznyak I, Foldi M, Szabo, G: Lymphatics and lymph circulation. Pergamon Press, New York:1967, p 155–158
Langhammer H, Bull U, Pfeiffer KJ, Hor G, Pabst HW: Experimental studies on the lymphatic drainage of the peritoneal cavity using 198Au-colloid. Lymphology 6: 149–157, 1973
Moghimi SM, Patel HM: Altered tissue-specific opsonic activities and opsonorecognition of liposomes in tumour-bearing rats. Biochim Biophys Acta 1285: 56–64, 1996
Gabizon A, Barenholz Y, Bialer M: Prolongation of the circulation time of doxorubicin encapsulated in liposomes containing a polyethylene glycol-derivatized phospholipid: pharmacokinetic studies in rodents and dogs. Pharm Res 10: 703–708, 1993
Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y: Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res 54: 987–992, 1994
Ñberall F, Oberhuber H, Maly K, Zaknun J, Demuth L, Grunicke H: Hexadecylphosphocholine inhibits inositol phosphate formation and protein kinase C activity. Cancer Res 51: 807–812, 1991
Kaufmann-Kolle P, Drevs J, Berger MR, Kötting J, Marschner N, Unger C, Eibl H: Pharmacokinetic behavior and antineoplastic activity of liposomal hexadecylphosphocholine. Cancer Chemother Pharmacol 34: 393–398, 1994
Kötting J, Marschner N, Neumüller W, Unger C, Eibl H: Hexadecylphosphocholine and octadecyl-methyl-glycero-3-phosphocholine: a comparison of hemolytic activity, serum binding and tissue distribution. In: Eibl H, Hilgard P, Unger C (eds) Progress in experimental tumor research. S. Karger, Basel, 1992, Vol 34, pp 131–142
Unger C, Fleer E, Damenz W, Hilgard P, Nagel G, Eibl, H: Hexadecylphosphocholine: determination of serum concentrations in rats. J Lipid Mediators 3: 71–78, 1991
Blume G, Cevc G: Liposomes for the sustained drug release in vivo. Biochim Biophys Acta 1029: 91–97, 1990
Mayhew E, Cimino M, Klemperer J, Lazo R, Wiemikowski J, A rbuck S: Free and liposomal doxorubicin treatment of intraperitoneal colon 26 tumor: therapeutic and pharmacologic studies. Sel Cancer Ther 6: 193–209, 1990
Rippe B: A three-pore model of peritoneal transport. Perit Dial Int 13: 35–38, 1993
Parker RJ, Hartman KD, Sieber SM: Lymphatic absorption and tissue disposition of liposome-entrapped [14C]adriamycin following intraperitoneal administration to rats. Cancer Res 41: 1311–1317, 1981
Nagel JD, Storm G, Varossieau FJ, Crommelin DJA, McVie JG: Intraperitoneal administration of mitoxantrone liposomes provides advantageous pharmacokinetics in comparison to i.v. or i.p. administration of free drug: a study in a rat model. Regional Cancer Treat (Germany) 9: 175–180, 1996
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Arndt, D., Zeisig, R., Fichtner, I. et al. Pharmacokinetics of sterically stabilized hexadecylphosphocholine liposomes versus conventional liposomes and free hexadecylphosphocholine in tumor-free and human breast carcinoma bearing mice. Breast Cancer Res Treat 58, 71–80 (1999). https://doi.org/10.1023/A:1006224611505
Issue Date:
DOI: https://doi.org/10.1023/A:1006224611505