Abstract
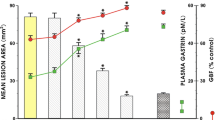
To elucidate the mechanism of intragastric nicotine protection against ethanol-induced gastric mucosal injury seen in a previous report and in our preliminary study, the following studies were performed. Rats were pretreated with naloxone (8 mg/kg intraperitoneal, 0.5 hr prior to study) to block opiate receptors; or capsaicin (125 mg/kg subcutaneous 10 days prior to study) to denervate the afferent sensory fibers; or indomethacin (2.5 mg/kg intragastric or 5 mg/kg subcutaneous, 1 hr prior to study) to inhibit endogenous prostaglandin synthesis. At 1-hr intervals, nicotine (4 mg/kg) or vehicle and 40% ethanol were then given intragastrically. Total gastric corpus mucosal lesion length was measured unbiasedly. In separate studies, gastric mucosal blood flow (GMBF) was assessed by hydrogen gas clearance before and after intragastric nicotine or vehicle; luminal mucus volume, gastric juice volume, and acid output were measured 1 hr after either intragastric nicotine or vehicle administration. The results showed that the acute protective effect of intragastric nicotine was associated with a significantly larger luminal mucus volume. It was not blocked by naloxone, capsaicin, or indomethacin. There was no increase in GMBF. The larger gastric residual volume did not account for the protection. We conclude that the mechanism mediating nicotine protection is unique and is independent of opiate receptors, capsaicin-sensitife afferent sensory nerve fibers, endogenous prostaglandin generation, or dilution of the injurious agent. The increase in luminal gastric mucus volume may contribute to the protective effect of intragastric nicotine against gastric mucosal injury produced by 40% ethanol.
Similar content being viewed by others
References
Wong SH, Ogle CW, Cho CH: The influence of chronic or acute nicotine pretreatment on ethanol-induced gastric ulceration in the rat. J Pharm Pharmacol 38:537–540, 1986
Arrigo-Reina R, Ferri S: Evidence of an involvement of the opioid peptidergic system in the reaction to stressful conditions. Eur J Pharmacol 64:85–88, 1980
Ferri S, Arrigo-Reina R, Candeletti S, Costa G, Murari G, Speroni E, Scoto G: Central and peripheral sites of action for the protective effect of opioids of the rat stomach. Pharmacol Res Commun 15:409–418, 1983
Glavin GB, Kiernan K, Hnatowich MR, Labella FS: Effects of morphine and naloxone on stress ulcer formation and gastric acid secretion. Eur J Pharmacol 124:121–127, 1986
Ferri S, Speroni E, Candeletti S, Cavicchini E, Romualdi P, Govani P, Marchini M: Protection by opioids against gastric lesions caused by necrotizing agents. Pharmacology 36:140–144, 1988
Szolcsányi J, Barthó L: Impaired defense mechanism to peptic ulcer in the capsaicin-desensitized rat.In Advances in Physiological Sciences, Vol. 29, Gastrointestinal Defence Mechanisms. G Mózsik, O Hänninen, T Jávor (eds). Budapest, Pergamon Press and Akadémiai Kiadó, 1981, pp. 39–51
Evangelista S, Maggi CA, Meli A: Evidence for a role of adrenals in the capsaicin-sensitive “gastric defence mechanism” in rats. Proc Soc Exp Biol Med 182:568–569, 1986
Holzer P, Sametz W: Gastric mucosal protection against ulcerogenic factors in the rat mediated by capsaicin-sensitive afferent neurons. Gastroenterology 91:975–981, 1986
Holzer P, Lippe IT: Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience 27:981–987, 1988
Holzer P, Pabst MA, Lippe IT: Intragastric capsaicin protects against aspirin-induced lesion formation and bleeding in the rat gastric mucosa. Gastroenterology 96:1425–1433, 1989
Robert A, Nezamis JE, Lancaster C, Hanchar AJ: Cytoprotection by prostaglandins in rats. Prevention of gastric necrosis produced by alcohol. HCl, NaOH, hypertonic NaCl, and thermal injury. Gastroenterology 77:433–443, 1979
Robert A, Nezamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ. Mild irritants prevent gastric necrosis through “adaptive cytoprotection” mediated by prostaglandins. Am J Physiol 245 (Gastrointest Liver Physiol 8):G113-G121, 1983
Cheung LY: Gastric mucosal blood flow: its measurement and importance in mucosal defense mechanisms. J Surg Res 36:282–288, 1984
Guth PH, Leung FW: Physiology of the gastric circulation.In Physiology of the Gastrointestinal Tract. 2nd ed. LR Johnson (ed). New York: Raven Press, 1987, pp 1031–1053.
Allen A: Structure and function of gastrointestinal mucus.In Physiology of the Gastrointestinal Tract. LR Johnson (ed). New York: Raven Press, 1981, pp. 617–639
Allen A, Carroll NJH: Adherent and soluble mucus in the stomach and duodenum. Dig Dis Sci 30(suppl):55S-62S, 1985
Lo SK, Leung FW, Guth PH: Protection against absoluteethanol-induced gastric antral and corpus mucosal injury: a gross and histologic study. Dig Dis Sci 33:1403–1408, 1988
Jaffe JH, Martin WR: Optioid analgesics and antagonists.In Goodman and Gilman's The Pharmacological Basis of Therapeutics, 7th ed. AG Gilman, LS Goodman, TW Rall, F Murad (eds). New York, Macmillan, 1985, pp 491–531
Gamse R: Capsaicin and nociception in the rat and mouse. Naunyn-Schmiedeberg's Arch Pharmacol 320:205–216, 1982
Vane JR: Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature (London) New Biol 231:232–235, 1971
Ligumsky M, Hansen DG, Kauffman GL Jr: Salicylic acid blocks indomethacin- and aspirin-induced cyclo-oxygenase inhibition in rat gastric mucosa. Gastroenterology 83:1043–1046, 1982
Pique JM, Leung FW, Tan HW, Livingston E, Scremin OU, Guth PH: Gastric mucosal blood flow response to stimulation and inhibition of gastric acid secretion. Gastroenterology 95:642–650, 1988
Leung FW, Guth PH, Scremin OU, Golanska EM, Kauffman GL Jr: Regional gastric mucosal blood flow measurements by hydrogen gas clearance in the anesthetized rat and rabbit. Gastroenterology 87:28–36, 1984
Leung FW, Morishita T, Livingston EH, Reedy T, Guth PH: Reflectance spectrophotometry for the assessment of gastroduodenal mucosal perfusion. Am J Physiol 252 (Gastrointest Liver Physiol 15):G797-G804, 1987
Livingston EH, Reedy T, Leung FW, Guth PH: Computerized curve fitting in the analysis of hydrogen gas clearance curves. Am J Physiol 257 (Gastrointest Liver Physiol 20):G668-G675, 1989
Polak JM, Bloom SR, Sullivan SN, Facer P, Pearse AG: Enkephalin-like immunoreactivity in the human gastrointestinal tract. Lancet 1(8019):972–974, 1977
Feurle GE, Helmstaedter V, Weber U: Met- and Leuenkephalin immuno- and bio-reactivity in human stomach and pancreas. Life Sci 31:2961–2969, 1982
Linnoila RI, DiAugustine RP, Miller RJ, Chang KJ, Cuatrecasas P: An immunohistochemical and radioimmunological study of the distribution of [Met5]- and [Leu5]- enkephalin in the gastrointestinal tract. Neuroscience 3:1187–1196, 1978
Ho MM, Dai S, Ogle CW: Decreased acid secretion and gastric lesion production by morphine in rats. Eur J Pharmacol 102:117–121, 1984
Gyires K, Fürst S, Farczádi E, Márton A: Morphine potentiates the gastroulcerogenic effect of indomethancin in rats. Pharmacology 30:25–31, 1985
Till M, Gáti T, Rábai K, Szombath D, Székely JI: Effect of [D-Met2. Pro5]enkephalinamide on gastric ulceration and transmucosal potential difference. Eur J Pharmacol 150:325–330, 1988
Esplugues JV, Whittle BJR: Morphine potentiation of ethanol-induced gastric mucosal damage in the rat. Role of local sensory afferent neurons. Gastroenterology 98:82–89, 1990
Buck SH, Burks TF: The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev 38:179–226, 1986
Gamse R, Leeman SE, Holzer P, Lembeck F: Differential effects of capsaicin on the content of somatostatin, substance P, and neurotensin in the nervous system of the rat. Naunyn-Schmiedeberg's Arch Pharmacol 317:140–148, 1981
Cheung LY, Sonnenschein LA: Measurement of regional gastric mucosal blood flow by hydrogen gas clearance. Am J Surg 147:32–37, 1984
Woods EF, Richardson JA: A survey of agents producing cardiovascular manifestations of epinephrine discharge. J Pharmacol Exp Ther 114:445–452, 1955
Florey H: Mucin and the protection of the body. Proc R Soc London Ser B 143:147–158, 1955
Ivey KJ, Triggs EJ: Absorption of nicotine by the human stomach and its effect on gastric ion fluxes and potential difference. Am J Dig Dis 23:809–814, 1978
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Endoh, K., Baker, M. & Leung, F.W. Mechanism of intragastric nicotine protection against ethanol-induced gastric injury. Digest Dis Sci 36, 39–46 (1991). https://doi.org/10.1007/BF01300085
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01300085