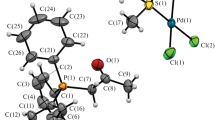
Abstract
Under solvothermal conditions and in the presence of oxovanadium species. organophosphonates undergo self-condensation reactions or condensation with phosphate to yield pyrodiphosphonate {RP(O)2OP(O)2 R}2, or organophos-phonatophosphate units {RP(O)2OPO3}3-. In this fashion, the reactions of (Ph4P)[VO2Cl2] withRPO3H2 (R=CH3,Ph) and Et3N in CH3CN at 110°C yielded (Ph4P)2 [VO{RP(O)2P(O)2 R}2](R = CH3(1), Ph (2)). However, the reaction of {Ph4P}[VO2Cl2],t-BuPO3H2 and (n-Bu4N)H2PO4 in acetonitrile at 125°C produced an unusual mixed valence V(IV)/V(III) cluster (Ph4P)2 (n-Bu4N)[(VO)6V{Me3CP(O)2OPO3}6] ⊎3CH3CN (3). Compounds1 and2 exhibit mononuclear molecular anions with the V(IV) center in the common square pyramidal coordination mode. The organodiphosphonate ligands adopt a bidentate coordination mode. The molecular anion of3 consists of a shell constructed of six V(IV) square pyramids linked by pentadentate {Me3CP(O)2OPO3}3- groups. Each organophosphonatophosphate ligand bridges four {VO5}square pyramids of the shell and directs the fifth oxygen donor toward the interior of the cluster, so as to bond to an octahedral V(III) located at the center of the cluster cavity. Crystal data:1, C26H26O5.5P3V0.5: triciinic Pl,a=10.836(2)A,b=ll.418(2)A,c=11.486(2)A,α=82.58(2)°,ß=75.29(2)°,γ=75.61(2)°,V=1328.1(7)A3,Z=2, Dcalc=l.362gcm-3;2, C36H32O6.5P3V0.5: monoclinic P21/c,a=12.823(3)A,b=14.318(3)A,c=18.581(4)A,ß=94.76(3)°,V=3999.7(13)A3,Z=4, Dcalc=l.342gcm-3 3, C94H139N4O42P14V7, triclinic Pl,a=13.589(3)A,b=17.835(4)A,c=38.915(8)A,α=81.64(2)°,ß=81.58(2)°,γ=82.87(2)°,V=9180(3)A3,Z=3, Dcalc=l.512gcm-3.
Similar content being viewed by others
References
M. I. Khan and J. Zubieta (1995).Prog. lnorg. Chem. 43, 1.
A. J. Jacobson,in A. K. Cheetham and P. Day (eds.)Solid State Chemistry (Clarendon Press, Oxford, 1992), pp. 182–230.
G. H. Huan A. J. Jacobson, J. W. Johnson, E. W. Corcoran, Jr. (1990).Chem. Mater.2, 2; J. W.,Johnson, A. J. Jacobson, W. M. Butler, and S. E. Rosenthal, J. E. Brody, and J. T. Levandowski (1989)J. Am. Chem. Soc,11, 38l.
M. I. Khan, Y.-S. Lee, C J. O'Connor, R. S,Haushalter, and J. Zubieta (1994).Inorg. Chem. 33. 3855.
M. I. Khan, Y.-S. Lee, C. J. O'Connor, R. S. Haushalter, and I. Zubieta (1994).J. Am. Chem. Soc. 116 4525.
M. I. Khan, Y.-S. Lee, C. J. O'Connor, R. S. Haushalter, and J. Zubieta (1994).Chem. Mater. 6. 721.
G. Huan, J. W,Johnson, A. J, Jacobson, and J. S. Merola (1990)J. Solid Stare Chem. 89 220
V. Soghomonian, Q. Chen, R. C. Haushalter, and J. Zubieta (1995).Angew. Chem. Int. Ed. Engl. 34 223
V. Soghomonian, R. Diaz, R. C. Haushalter, C. J. O'Connor, and J, Zubieta (1995).Inorg. Chem. 34 4460.
V. Soghomoniam, R. C. Haushalter, and J. Zubieta (1995).Chem. Mater. 7 1648.
Q. Chen, J. Salta, and J. Zubieta (1993).Inorg. Chem. 32 4485.
Q. Chen and J. Zubieta (1994).J. Chem. Soc. Chem. Commun. 2663.
Q. Chen and J. Zubieta (1993).Angew. Chem. lnt. Ed. Engl. 32, 261.
J. Salta, Q. Chen, Y.-D. Chang, and J. Zubieta (1994).Angew. Chem. Int. Ed. Engl. 33, 757.
Y.-D. Chang, J. Salta and J. Zubieta (1994).Angew. Chem. Int. Ed. Engl. 33, 325.
Q. Chen and J. Zubieta (1994).J. Chem. Soc. Chem. Commun. 1635.
A. Müller, K. Hovemeier, E. Krichemeyer, and H. Bögge (1995).Angew. Chem., Int. Ed. Engl. 34, 779; A. Müller, K. Hovemeier, and R. Rohlfing (1992).Angew. Chem. Int. Ed. Engl. 31, 1192.
M. I. Khan and J. Zubieta (1994).Angew. Chem. Int. Ed. Engl. 33, 760.
G. H. Huan, A. J. Jacobson, and V. W. Day (1991).Angew. Chem. Int. Ed. Engl. 30. 422.
D. Fenske, A.-F. Shihada, H. Schwab, and K. Dehnicke (1986).Z. Anorg. Allgem. Chem. 471, 140
N. Walker and D. Stuart (1983).Acta Crystallogr. Sect. 39, 158.
teXsan: Texray Structural Analysis Package, Revised (Molecular Structure Corporation, The Woodlands, TX 1992).
D. T. Cromer and J. T. Waber,International Tables for X-Ray Crystallography, Vol. IV (Kynoch Press, Birmingham, England, 1974).
D. C. Creagh and J. W. McAuley,International Tables for X-Ray Crystallography, Vol. C. Table 4, 2, 6, 8. (Kluwer Academic, Boston 1992)
SHELXTL PCRM, Siemens Analytical X-Ray Instruments, Inc. (Madison, WI, 1990).
M. I. Khan and J. Zubieta (1995).Prog. Inorg. Chem. 43, 1.
M. I. Khan, Q. Chen, H. Höpe, S. Parkin, C. J. O'Connor, and J. Zubieta (1993).Inorg. Chem. 32, 2929.
M. I. Khan, Q. Chen, D. P. Goshorn, H. Höpe, S. Parkin, and J. Zubieta (1992).J. Am. Chem. Soc. 114, 3341.
M. I. Khan, Y.-S. Lee, C. J. O'Connor, and J. Zubieta (1994).J. Am. Chem. Soc. 116, 5001.
M. I. Khan and J. Zubieta (1992).J. Am. Chem. Soc. 114, 10058.
Y.-D. Chang and J. Zubieta (1996).Inorg. Chim. Acta, in press.
I. D. Brown,in M. O'Keefe and A. Navrotsky (eds.),Structure and Bonding in Crystals (Academic Press, New York, 1981), pp. 1–30.
J. Salta, Y.-D. Chang, and J. Zubieta (1994). J. Chem. Soc., Chem. Commun. 1039.
L. Benhameda, A. Grandin, M. M. Borel, A. LeClaire, and B. Raveau (1992).J. Solid State Chem. 97, 131.
S.-J. Hevu, R. I. Carroll, and D. L. Serra (1994).J. Solid State Chem. 110, 290.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Salta, J., Zubieta, J. Studies of the oxovanadium-organophosphonate system: Formation of pyrodiphosphonate and pyrophosphophosphonate units through metal-mediated ligand condensations. The crystal and molecular structures of (Ph4P)2[VO{RP(O)2OP(O)2 R}2] (R=Me, Ph) and (Ph4P)2(n-Bu4N)[(VO)6V{t-BuP(O)2OPO3}6]. J Clust Sci 7, 531–551 (1996). https://doi.org/10.1007/BF01165800
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF01165800