Abstract
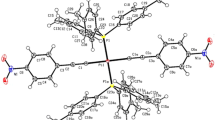
Reactions PtI2L (L = P,P-bonded 1,2-bis ((2-pyridyl)phosphino(ethane, d(py)pe, or 1,2-bis ((2-pyridyl)phosphino)cyclopentane, d(py)pcp) with excess AgNO3 in acetic acid/ethanol afford the polymers [Pt2(d(py)pe)2Ag4(NO3)8(H2O)2]n(1 ) and [Pt2(d(py)pcp)2Ag6(NO3)10] n (2), respectively, which contain Pt2L 4+2 cores with each L ligand nowP,N-bridging two Pt-atoms; these Pt2 cores are connected via bridging AgNO3 clusters, the Ag being bound to the pyridyl-N or nitrate-O atoms. X-ray crystallographic data reveal Pt–Pt contacts of ∼2.76 Å indicative of bonding.
Similar content being viewed by others
REFERENCES
W. A. Fordyce, J. G. Brummer, and G. A. Crosby (1981). J. Am. Chem. Soc. 103, 7061; H. Isci and W. R. Mason (1985). Inorg. Chem. 24, 1761; C.-M. Che, L. G. Butler, and H. B. Gray (1981). J. Am. Chem. Soc. 103, 7796.
C. Bellitto, A. Flamini, L. Gastaldi, and L. Scaramuzza (1983). Inorg. Chem. 22, 444; T. G. Appleton, K. A. Byriel, J. R. Hall, C. H. L. Kennard, and M. T. Mathieson (1992). J. Am. Chem. Soc. 114, 7305; R. El-Mehdawi, F. R. Fronczek, and D. M. Roundhill (1986). Inorg. Chem. 25, 1155.
F. A. Cotton, J. H. Matonic, and C. A. Murillo (1996). Inorg. Chem. 35, 498; idem. (1997). Inorg. Chim. Acta 264, 61.
E. Zangrando, F. Pichierri, L. Randaccio, and B. Lippert (1996). Coord. Chem. Rev. 156, 275.
J. A. R. Navarro, M. A. Romero, J. M. Salas, M. Quirs, J. El Bahraoui, and J. Molina (1996). Inorg. Chem. 35, 7829.
O. Simonsen and H. Toftlund (1981). Inorg. Chem. 20, 4044.
C. Bellitto, A. Flamini, O. Piovessana, and P.F. Zanazzi (1980). Inorg. Chem. 19, 3632.
M. A. Filomena Dos Remedios Pinto, P. J. Sadler, S. Neidle, M. R. Sanderson, and A. Subbiah (1980). J. Chem. Soc., Chem. Comun., 13.
Y. Xie and B. R. James (1991). J. Organomet. Chem. 417, 277; Y. Xie, C. L. Lee, Y. Yang, S. J. Rettig, and B. R. James (1992). Can. J. Chem. 70, 751.
I. R. Baird, M. B. Smith, and B. R. James (1995). Inorg. Chim. Acta 235, 291.
K. S. MacFarlane, N. D. Jones, R. P. Schutte, M. B. Smith, S. J. Rettig, and B. R. James. To be published (details also available from the authors).
J. X. McDermott, J. F. White, and G. M. Whitesides (1976). J. Am. Chem. Soc. 98, 6521.
teXsan: Crystal structure analysis package, Unix version 1.8, Molecular Structure Corporation, The Woodlands, TX, U.S.A., 1995; and d* TREK: Area Detector Software, Version 3.8, Molecular Structure Corporation, The Woodlands, TX, U.S.A., 1997.
International Tables for X-Ray Crystallography, Vol IV (Kynoch Press, Birmingham, UK, 1974) (Present distributor: Kluwer Academic Publishers, Boston, MA, USA) pp. 99–102; International Tables for Crystallography, Vol. C (Kluwer, 1992) pp. 200–206.
G. Bandoli, P. A. Caputo, F. P. Intini, M. F. Sivo, and G. Natile (1997). J. Am. Chem. Soc. 119, 10370.
M. P. Brown, S. J. Cooper, A. A. Frew, L. M. Manojlovic, K. W. Muir, R. J. Puddephatt, K. R. Seddon, and M. A. Thomson (1981). Inorg. Chem. 20, 1500.
F. A. Cotton, M. Matusz, R. Poli, and X. Feng (1988). J. Am. Chem. Soc. 110, 1144.
J. J. Novoa, G. Aullón, P. Allemany, and S. Alvarez (1995). J. Am. Chem. Soc. 117, 7169.
C. Mealli, F. Pichierri, L. Randaccio, E. Zangrando, M. Krumm, D. Holtenrich, and B. Lippert (1995). Inorg. Chem. 34, 3418.
L. Pauling, The Nature of the Chemical Bond, 3rd Ed. (Cornell University Press, Ithaca, N.Y., 1960) p. 255; idem. (1947). J. Am. Chem. Soc. 69, 542.
A. H. Reis, Jr. and S. W. Peterson (1976). Inorg. Chem. 15, 3187.
The H6 protons (adjacent to N in the o-pyridyl group) generally appear downfield from other protons in the phenyl region of NMR spectra and have a distinct identifiable pattern, appearing as approximate doublets (actually multiplets). H. J. Jackobsen (1970). J. Mol. Spectrosc. 34, 245; G. E. Griffin and W. A. Thomas (1970). J. Chem. Soc. (B) 477; R. P. Schutte, S. J. Rettig, A. M. Joshi, and B. R. James (1997). Inorg. Chem. 36, 5809.
R. I. Lancashire, in Comprehensive Coordination Chemistry, Vol. 5 (G. Wilkinson, R. D. Gillard, and J. A. McCleverty, eds.) (Pergamon Press, Oxford, 1987), p. 786.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jones, N.D., Rettig, S.J. & James, B.R. Platinum(II)-Silver(I) Polymers Bearing Platinum(II) Clusters Containing Chelated P,P- and Bridged P, N-2-Pyridylphosphine Ligands. Journal of Cluster Science 9, 243–258 (1998). https://doi.org/10.1023/A:1022622825888
Issue Date:
DOI: https://doi.org/10.1023/A:1022622825888