Abstract
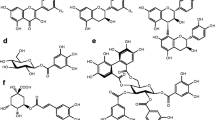
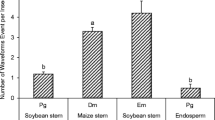
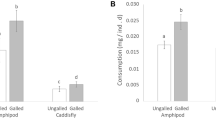
We examined several of the mechanisms that have been reported to enable polyphagous grasshoppers (Orthoptera: Acrididae) to tolerate ingested hydrolyzable tannins: hydrolysis, adsorption on the peritrophic envelope, and peritrophic envelope impermeability. None of these mechanisms explain the tolerance ofMelanoplus sanguinipes to ingested tannic acid. In this species, tannin hydrolysis was 12–47% complete, adsorption accounted for less than 1% of the tannic acid contained in the midgut, and the peritrophic envelope was permeated by several gallotannins. The foregut is the main site for the chemical transformation of tannic acid in this species. InPhoetaliotes nebrascensis, hydrolysis was more extensive (82% complete), but the peritrophic envelope was readily permeated by two gallotannins. Oxidizing redox conditions were found in the guts of both species, and ingested tannins were oxidized inM. sanguinipes. We hypothesize that the tolerance of some polyphagous grasshoppers to ingested hydrolyzable tannins may be the consequence of their ability to tolerate the reactive oxygen species generated by polyphenol oxidation, whereas others may rely on rapid and extensive hydrolysis.
Similar content being viewed by others
References
Ahmad, S. 1995. Antioxidant mechanisms of enzymes and proteins, pp. 238–272,in S. Ahmad (ed.). Oxidative Stress and Antioxidant Defenses in Biology. Chapman and Hall, New York.
Appel, H. M. 1993. Phenolics in ecological interactions: the importance of oxidation.J. Chem. Ecol. 19:1521–1552.
Appel, H. M., andMartin, M. M. 1990. Gut redox conditions in herbivorous lepidopteran larvae.J. Chem. Ecol. 16:3277–3290.
Barbehenn, R. V., andMartin, M. M. 1992. The protective role of the peritrophic membrane in the tannin-tolerant larvae ofOrgyia leucostigma (Lepidoptera).J. Insect Physiol. 38:973–980.
Barbehenn, R. V., andMartin, M. M. 1994. Tannin sensitivity in larvae ofMalacosoma disstria (Lepidoptera): roles of the peritrophic envelope and midgut oxidation.J. Chem. Ecol. 20:1985–2001.
Bernays, E. A. 1978. Tannins: an alternative viewpoint.Entomol. Exp. Appl. 24:244–253.
Bernays, E. A., andChamberlain, D. J. 1980. A study of tolerance of ingested tannin inSchistocerca gregaria.J. Insect Physiol. 26:415–420.
Bernays, E. A., Chamberlain, D., andMcCarthy, P. 1980. The differential effects of ingested tannic acid on different species of Acridoidea.Entomol. Exp. Appl. 28:158–166.
Bernays, E. A., andSimpson, S. J. 1989. Nutrition. pp. 105–128,in R. F. Chapman and A. Joern (eds.). Biology of Grasshoppers. John Wiley and Sons, New York.
Bignell, D. E. 1984. Direct potentiometric determination of redox potentials of the gut contents in the termitesZootermopsis nevadensis andCubitermes severus and in three other arthropods.J. Insect Physiol. 30:169–174.
Cadenas, E. 1995. Mechanisms of oxygen activation and reactive oxygen species detoxification. pp. 1–61.in S. Ahmad (ed.). Oxidative Stress and Antioxidant Defenses in Biology. Chapman and Hall, New York.
Chapman, R. F. 1982. The Insects: Structure and Function. Harvard University Press, Cambridge, MA.
Cilliers, J. J. L., andSingleton, V. L. 1989. Nonenzymic autoxidative phenolic browning reactions in a caffeic acid model system.J. Agric. Food Chem. 37:390–396.
Dadd, R. H. 1970. Digestion in insects. pp. 117–145.in M. Florkin and B. T. Scheer (eds.), Chemical Zoology. Academic Press, New York.
Droste, H. J., andZebe, E. 1974. Carbohydrasen und Kohlenhydratverdauung im Darmtrakt vonLocusta migratoria.J. Insect Physiol. 20:1639–1657.
Evans, W. A. L., andPayne, D. W. 1964. Carbohydrases of the alimentary tract of the desert locust,Schistocerca gregaria Forsk.J. Insect Physiol. 10:657–674.
Felton, G. W. 1995. Oxidative stress of vertebrates and invertebrates. pp. 356–434,in S. Ahmad (ed.). Oxidative Stress and Antioxidant Defenses in Biology. Chapman and Hall, New York.
Felton, G. W., andSummers, C. 1995. Antioxidant systems in insects.Arch. Insect Biochem. Physiol. 29:187–197.
Gangwere, S. K., Evans, F. C., andNelson, M. L. 1976. The food-habits and biology of Acrididae in an old-field community of southeastern Michigan.Great Lakes Entomol. 9:83–123.
Hagerman, A. E., Robbins, C. T., Weerasuriya, Y., Wilson, T. C., andMcArthur, C. 1992. Tannin chemistry in relation to digestion.J. Range Manage. 45:57–62.
Hathway, D. E., andSeakins, J. W. T. 1957. Autoxidation of polyphenols. Part 3. Autoxidation in neutral aqueous solution of flavans related to catechin.J. Chem. Soc. 2:1562–1566.
House, H. L. 1974. Digestion. pp. 63–117,in M. Rockstein (ed.). The Physiology of Insecta. Academic Press, New York.
Igarashi, K., andYasui, T. 1985. Oxidation of free methionine and methionine residues in protein involved in the browning reaction of phenolic compounds.Agric. Biol. Chem. 49:2309–2315.
Isely, F. B. 1944. Correlation between mandibular morphology and food specificity in grasshoppers.Ann. Entomol. Soc. Am. 37:47–67.
Joern, A. 1983. Host plant utilization by grasshoppers (Orthoptera: Acrididae) from a sandhills prairie.J. Range Manage. 36:793–797.
Leatham, G. F., King, V., andStahmann, M. A. 1980. In vitro protein polymerization by quinones or free radicals generated by plant or fungal oxidative enzymes.Phytopathology 70:1134–1140.
Maddrell, S. H. P., andGardiner, B. O. C. 1980. The permeability of the cuticular lining of the insect alimentary canal.J. Exp. Biol. 85:227–237.
Mole, S., andJoern, A. 1994. Feeding behavior of graminivorous grasshoppers in response to host-plant extracts, alkaloids, and tannins.J. Chem. Ecol. 20:3097–3109.
Okuda, T., Yoshida, T., andHatano, T. 1993. Classification of oligomeric hydrolysable tannins and specificity of their occurrence in plants.Phytochemistry 32:507–521.
Price, R. G., andRobinson, D. 1966. A comparison of theβ-D-glucosidase andβ-D-galactosidase activities from eleven enzyme sources.Comp. Biochem. Physiol. 17:129–138.
Raubenheimer, D. 1992. Tannic acid, protein, and digestible carbohydrate: dietary imbalance and nutritional compensation in locusts.Ecology 73:1012–1027.
Robinson, D. 1964. Fluorimetric determination of glycosidases in the locust (Locusta migratoria) and other insects.Comp. Biochem. Physiol. 12:95–105.
Steinly, B. A., andBerenbaum, M. 1985. Histopathological effects of tannins on the midgut epithelium ofPapilio polyxenes andPapilio glaucus.Entomol. Exp. Appl. 39:3–9.
Summers, C. B., andFelton, G. W. 1994: Prooxidant effects of phenolic acids on the generalist herbivoreHelicoverpa zea (Lepidoptera: Noctuidae): potential mode of action for phenolic compounds in plant anti-herbivore chemistry.Insect Biochem. Molec. Biol. 24:943–953.
Thomson, R. H. 1962. Some naturally occurring black pigments. pp. 99–113,in T. S. Gore, B. S. Joshi, S. V. Sunthankar, and B. D. Tilak (eds.). Recent Progress in the Chemistry of Natural and Synthetic Colouring Matters and Related Fields. Academic Press, New York.
Wilkinson, L. 1990. SYSTAT: the System for Statistics. SYSTAT Inc., Evanston, IL.
Zhao, Y. 1995. Simultaneous determination of hydrolyzable tannin and condensed tannin. M.S. Thesis, Miami Univ., Oxford, Ohio.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barbehenn, R.V., Martin, M.M. & Hagerman, A.E. Reassessment of the roles of the peritrophic envelope and hydrolysis in protecting polyphagous grasshoppers from ingested hydrolyzable tannins. J Chem Ecol 22, 1901–1919 (1996). https://doi.org/10.1007/BF02028511
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02028511