Abstract
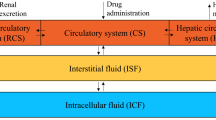
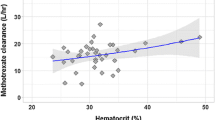
The effects of plasma concentration and pH on the steady-state volume of distribution, Vss,of methotrexate (MTX) were studied in five conditioned male beagle-mongrel dogs. Steady-state plasma MTX concentrations of approximately 1, 20, and 100μg/ml were targeted for by i.v. bolus doses followed by i.v. infusions. An isotonic solution of sodium bicarbonate or ammonium chloride was simultaneously infused for the purpose of inducing plasma pH change, while the infusion of an isotonic solution of sodium chloride served as a control. Plasma and urine concentrations of MTX were quantitated by a sensitive high-performance liquid chromatographic method, and the Vss of MTX was estimated by a recently reported physiologically based method of Chiou and Lam. Statistically significant (p<0.05) concentration and plasma pHdependent Vss of MTX were observed. Concentration dependence of Vss was noted in sodium chloride and ammonium chloride infused dogs, but not in bicarbonate treated dogs. There was an average 50.0 and 44.8% increase in Vss at 1 μg/ ml relative to the two higher concentrations (20 and 100 μg/ ml) for dogs treated with ammonium and sodium chloride, respectively. However, Vss of MTX at the targeted concentrations of 20 and 100 μg/ml was relatively constant. Plasma pHdependence of Vss was observed only at the plasma concentration of 1 μg/ml, and on the average, ammonium chloride and sodium chloride treatments resulted in 50.0 and 31.3% higher Vss,respectively, when compared with the bicarbonate treatment. These phenomena appear to be adequately explained by the reported tissue uptake kinetics of MTX.
Similar content being viewed by others
References
J. Jolivet, K. H. Cowan, G. A. Curt, N. J. Clendeninn, and B. A. Chabner. The pharmacology and clinical use of methotrexate.N. Engl. J. Med. 309:1094–1104 (1983).
D. A. Gewirtz, J. C. White, J. K. Randolph, and I. D. Goldman. Transport, binding, and polyglutamation of methotrexate in freshly isolated rat hepatocytes.Cancer Res. 40:573–578 (1980).
R. L. Schilsky, B. D. Bailey, and B. A. Chabner. Characteristics of membrane transport of methotrexate by cultured human breast cancer cells.Biochem. Pharmacol. 30:1537–1542 (1981).
F. M. Sirotnak, M. G. Sargent, and D. J. Hutchison. Genetically alterable transport of amethopterin in Diplococcus pneumoniae I. Physiological properties and kinetics of the wild-type system.J. Bacteriol. 93:309–314 (1967).
F. M. Sirotnak, S. Kurita, and D. J. Hutchison. On the nature of a transport alteration determining resistance to amethopterin in the L1210 leukemia.Cancer Res. 28:75–80 (1968).
W. B. Strum. ApH-dependent, carrier-mediated transport system for the folate analog, amethopterin, in rat jejunum.J. Pharmacol. Exp. Ther. 230:640–645 (1977).
R. G. Stoller, S. A. Jacobs, J. C. Drake, R. J. Lutz, and B. A. Chabner. Pharmacokinetics of high-dose methotrexate (NSC-740).Cancer Chemother. Rep. 6:19–24 (1975).
H. Breithaupt and E. Kuenzlen. Pharmacokinetics of methotrexate and 7-hydroxymethotrexate following infusions of high-dose methotrexate.Cancer Treat. Rep. 66:1733–1741 (1982).
G. H. Mudge. Agents affecting volume and composition of body fluids. In A. G. Gilman, L. S. Goodman, and A. Gilman (eds.),The Pharmacological Basis of Therapeutics, 6th ed. Macmillan, New York, 1980, p. 868.
W. L. Chiou and G. Lam. Simplified physiologically based method to estimate steady-state volume of distribution.J. Pharm. Sci. 70:967–968 (1981).
C. Y. Lui, M. G. Lee, and W. L. Chiou. UrinarypH and urine flow independent renal clearance of methotrexate in dogs, to be published.
M. G. Lee, M. L. Chen, S. M. Huang, and W. L. Chiou. Pharmacokinetics of drugs in blood I: Unusual distribution of gentamicin.Biopharm. Drug Dispos. 2:89–97 (1981).
M. G. Lee, C. Y. Lui, M. L. Chen, and W. L. Chiou. Pharmacokinetics in blood IV: Unusual distribution, storage effect and metabolism of methotrexate.Int. J. Clin. Pharmacol. Ther. Toxicol 22:530–537 (1984).
M. L. Chen and W. L. Chiou. Clearance studies of methotrexate and 7-hydroxymethotrexate in rabbits after multiple-dose infusion.J. Pharmacokin. Biopharm. 11:515–520 (1983).
M. L. Chen and W. L. Chiou. Sensitive and rapid high-performance liquid chromatographic method for the simultaneous determination of methotrexate and its metabolites in plasma, saliva and urine.J. chromatogr. Biomed. Appl. 226:125–134 (1981).
A. Higashi and L. Peters. A rapid colorimetric method for the determination of inulin in plasma and urine.J. Lab. Clin. Med. 35:475–482 (1950).
M. L. Chen, G. Lam, M. G. Lee, and W. L. Chiou. Arterial and venous blood sampling in pharmacokinetic studies: griseofulvin.J. Pharm. Sci. 71:1386–1389 (1982).
E. S. Henderson, R. H. Adamson, C. Denham, and V. T. Oliverio. The metabolic fate of tritiated methotrexate I. Absorption, excretion and distribution in mice, rats, dogs and monkeys.Cancer Res. 25:1008–1017 (1965).
D. G. Johns, S. Sperti, and S. V. Burgen. The metabolism of tritiated folic acid in man.J. Clin. Invest. 40:1684–1695 (1961).
G. M. Rubin, T. N. Tozer, and S. Øie. Concentration-dependence of salicylate distribution.J. Pharm. Pharmacol. 35:115–117 (1983).
J. W. Jailer, C. G. Zubrod, M. Rosenfield, and J. A. Shannon. Effect of acidosis and anoxia on the concentration of quinacrine and chloroquine in blood.J. Pharmacol. Exp. Ther. 92:345–351 (1948).
B. B. Brodie, L. C. Mark, E. M. Papper, P. A. Life, E. Bernstein, and E. A. Rovenstine. The fate of thiopental in man and a method for its estimation in biological material.J. Pharmacol. Exp. Ther. 98:85–96 (1950).
W. J. Waddell and T. C. Butler. The distribution and excretion of phenobarbital.J. Clin. Invest. 36:1217–1226 (1957).
M. L. Chen and W. L. Chiou. Pharmacokinetics of methotrexate and 7-hydroxymethotrexate in rabbits after i.v. administration.J. Pharmacokin. Biopharm. 11:499–513 (1983).
M. L. Chen, W. P. McQuire, T. E. Lad, and W. L. Chiou. A specific HPLC assay to determine the pharmacokinetics of methotrexate in patients.Int. J. Clin. Pharmacol Ther. Toxicol. 22:1–6 (1984).
A. J. Patterson, W. A. Ritschel, D. Zellner, and S. H. Kim. Methotrexate serum and saliva concentrations in patients.Int. J. Clin. Pharmacol. Ther. Toxicol. 19:381–385 (1981).
L. Kristensen, K. Weismann, and L. Hutters. Renal function and the rate of disappearance of methotrexate from serum.Eur. J. Clin. Pharmacol. 8:439–444 (1975).
D. Bratlid and P. J. Moe. Pharmacokinetics of high-dose methotrexate treatment in children.Eur. J. Clin. Pharmacol. 14:143–147 (1978).
C. F. Stewart, W. R. Crom, W. P. Bowman, P. R. Hutson, and W. E. Evans. Influence of serum concentration on renal clearance of methotrexate in children with acute lymphocytic leukemia.Drug Intell. Clin. Pharm. 16:482 (1982).
D. J. Greenblatt, D. R. Abernethy, and M. Divoll. Is volume of distribution at steady state a meaningful kinetic variable?J. Clin. Pharmacol. 23:391–400 (1983).
W. L. Chiou, C. Y. Lui, and G. Lam. Plasma area method in relative bioavailability evaluation of drugs with changing biological half-lives.J. Pharm. Sci. 70:109–112 (1981).
W. L. Chiou. The physiological significance of the apparent volume of distribution, Vdβ or Vdarea, in pharmacokinetic studies.Res. Commun. Chem. Pathol. Pharmacol. 33:499–508 (1981).
K. C. Huang, B. A. Wenczak, and Y. K. Liu. Renal tubular transport of methotrexate in the rhesus monkey and dog.Cancer Res. 39:4843–4848 (1979).
W. A. Bleyer. The clinical pharmacology of methotrexate.Cancer 41:36–51 (1978).
G. Lam and W. L. Chiou. Determination of the steady-state volume of distribution using arterial and venous plasma data from constant infusion studies with procainamide.J. Pharm. Pharmacol. 34:132–134 (1981).
W. L. Chiou, G. Lam, M. L. Chen, and M. G. Lee. Effect of arterial-venous plasma concentration difference on the determination of mean residence time of drugs in the body.Res. Commun. Chem. Pathol. Pharmacol. 35:17–26 (1982).
Author information
Authors and Affiliations
Additional information
This research was supported in part by a grant from the National Cancer Institute, CA-29754.
Abstracted from a dissertation submitted in 1984 by Chung Y. Lui to the Graduate College, University of Illinois at Chicago, in partial fulfillment of Doctor of Philosophy Degree requirements.
Rights and permissions
About this article
Cite this article
Lui, C.Y., Lee, M.G. & Chiou, W.L. Concentration andpH dependent steady-state volume of distribution of methotrexate estimated by a simple physiologically based method. Journal of Pharmacokinetics and Biopharmaceutics 12, 597–610 (1984). https://doi.org/10.1007/BF01059555
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01059555