Abstract
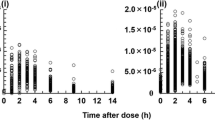
The pharmacokinetics of cyclosporine A (CyA) was studied in 21 uremic patients. The plasma concentrations after an oral dose and a subsequent short-term infusion were analyzed simultaneously by nonlinear regression. Bi- and triexponential disposition models with either zero- or first-order absorption were fitted to the data. A triexponential disposition model with zero-order absorption was generally found to best describe the concentration-time profile. The bioavailability and clearance were estimated to be 0.24±0.10 and 21±8 L/hr, respectively. These values differed only marginally from those predicted by the other models. Similar bioavailability estimates were also obtained from a three-compartment model where elimination was assumed saturable, from a deconvolution procedure, and from analyses based on blood concentrations. Markedly higher bioavailabilities (0.34±0.13) were obtained when a model-independent AUCcorrection procedure, commonly used to calculate CyA bioavailability, was used. The difference could not be explained by poor description of data in the model-dependent analyses, but rather by overestimation in the model-independent analyses mainly due to errors in the extrapolations used. Thus, by the simultaneous fitting procedure, which is a new approach for estimating CyA bioavailability, drawbacks of the AUCcorrection procedure could be avoided. Further, future studies of CyA bioavailability could be designed with a markedly shorter and more convenient length of time if analyzed by the proposed method.
Similar content being viewed by others
References
R. J. Ptachcinski, R. Venkataramanan, and G. J. Burckart. Clinical pharmacokinetics of cyclosporin.Clin. Pharmacokin. 11:107–132 (1986).
B. D. Kahan, M. Ried, and J. Newburger. Pharmacokinetics of cyciosporine in human renal transplantation.Transplant. Proc. 15:446–453 (1983).
L. Vernillet, B. Moulin, C. Dadoun, J. F. Le Bigot, and J. P. Fillastre. Pharmacokinetics of Cyciosporine A in patients with nephrotic syndrome.Transplant. Proc. 20:529–535 (1988).
K. L. Reynolds, J. Grevel, S. Y. Gibbons, M. S. Welsh, L. P. Rutzky, and B. D. Kahan. Cyciosporine pharmacokinetics in uremic patients: Influence of different assay methods.Transplant. Proc. 20:462–465 (1988).
R. J. Ptachcinski, R. Venkataramanan, J. T. Rosenthal, G. J. Burckart, R. J. Taylor, and T. R. Hakala. Cyciosporine pharmacokinetics in early course of renal transplantation.Clin. Pharmacol. Ther. 38:296–300 (1985).
K. Albrecht, W. Niebel, G. Marggraf, and F. W. Eigler, Cyciosporine pharmacokinetics in the early course of renal transplantation.Transplant. Proc. 19:1719 (1987).
R. J. Ptachcinski, R. Venkataramanan, J. T. Rosenthal, G. J. Burckart, R. J. Taylor, and T. R. Hakala. The effect of food on cyciosporine absorption.Transplantation 40:174–176 (1985).
R. J. Ptachcinski, G. J. Burckart, J. T. Rosenthal, R. Venkataramanan, D. L. Howrie, R. J. Taylor, E. D. Avner, D. Ellis, and T. R. Hakala. Cyciosporine pharmacokinetics in children following cadaveric renal transplanation.Transplant. Proc. 18:766–767 (1986).
A. Lindberg, B. Odlind, G. Tufveson, B. Lindström, and J. Gabrielsson, The pharmacokinetics of Cyciosporine A in uremic patients.Transplant. Proc. 18:144–152 (1986).
R. Venkataramanan, R. J. Ptachcinski, G. J. Burckart, J. Gray, D. H. Van Thiel, and T. E. Starzl. Cyciosporine bioavailability in liver disease.Drug Intell. Clin. Pharm. 19:451 (1985).
A. Johnston, J. T. Marsden, K. K. Hla, J. A. Henry, and D. W. Holt. The effect of vehicle on the oral absorption of cyclosporin.Br. J. Clin. Pharmacol. 21:331–333 (1986).
S. K. Gupta and L. Z. Benet. Absorption kinetics of cyclosporine in healthy volunteers.Biopharm. Drug Dispos. 10:591–596 (1989).
J. M. Tredger, N. V. Naoumov, C. M. Steward, J. G. O'Grady, J. Grevel, A. A. Niven, A. W. Kelman, B. Whiting, and R. Williams, Influence of biliary T tube clamping on cyciosporine pharmacokinetics in liver transplant recipients.Transplant. Proc. 20:512–515 (1988).
R. Venkataramanan, G. J. Burckart, R. J. Ptachcinski, A. Lee, R. L. Hardesty, and B. P. Griffith. Cyciosporine pharmacokinetics in heart transplant patients.Transplant Proc. 18:768–770 (1986).
B. D. Kahan. Individualization of cyciosporine therapy using pharmacokinetic and pharmacodynamic parameters.Transplantation 40:457–476 (1985).
S. K. Gupta, B. Legg, L. R. Solomon, R. W. G. Johnson, and M. Rowland. Pharmacokinetics of cyclosporin: influence of rate of constant intravenous infusion in renal transplant patients.Br. J. Clin. Pharmacol. 24:519–526 (1987).
R. J. Sawchuk and L. L. Cartier. Liquid-chromatographic determination of cyclosporin A in blood and plasma.Clin. Chem. 27:1368–1371 (1981).
G. Tufveson, B. Odlind, O. Sjöberg, A. Lindberg, J. Gabrielsson, B. Lindström, H. Lithell, I. Selinus, T. Tötterman, and J. Wahlberg. A longitudinal study of the pharmacokinetics of cyciosporine A and in vitro lymphocyte responses in renal transplant patients.Transplant. Proc. 18:16–24 (1986).
Statistical Consultants, Inc. PCNONLIN and NONLIN84: Software for the statistical analysis of nonlinear models.Am. Statist. 40:52 (1986).
C. M. Metzler. Extended least squares (ELS) procedures for pharmacokinetic models.J. Pharm. Sci. 76:565–571 (1987).
M. Gibaldi and D. Perrier.Pharmacokinetics, Marcel Dekker, New York, 1982.
K. Iga, Y. Ogawa, T. Yashiki, and T. Shimamoto. Estimation of drug absorption rates using a deconvolution method with nonequal sampling times.J. Pharmacokin. Biopharm. 14:213–225 (1986).
T. K. Daneshmend, L. Jackson, and C. J. C. Roberts. Physiological and pharmacological variability in estimated hepatic blood flow in man.Br. J. Clin. Pharmacol. 11:491–496 (1981).
K. Yamaoka, T. Nakagawa, and T. Uno. Application of Akaike's information criteria (AIC) in the evaluation of linear pharmacokinetic equations.J. Pharmacokin. Biopharm. 6:165–175 (1978).
J.-P. Reymond, J.-L. Steimer, and W. Niederberger. On the dose dependency of cyclosporin A absorption and disposition in healthy volunteers.J. Pharmacokin. Biopharm. 16:331–353 (1988).
J. Grevel, E. Nuesch, E. Abisch, and K. Katz. Pharmacokinetics of oral cyclosporin A (Sandimmun) in healthy subjects.Eur. J. Clin. Pharmacol. 31:211–216 (1986).
T. Beveridge, A. Gratwohl, F. Michot, W. Niederberger, E. Nuesch, K. Nussbaumer, P. Schaub, and B. Speck. Cyclosporin A: Pharmacokinetics after a single dose in man and serum levels after multiple dosing in recipients of allogenic bone-marrow grafts.Curr. Ther. Res. 30:5–18 (1981).
J. Newburger and B. D. Kahan. Cyclosporine pharmacokinetics in man.Transplant. Proc. 15:2413–2415 (1983).
F. Follath, M. Wenk, S. Vozeh, G. Thiel, F. Brunner, R. Loertscher, M. Lemaire, K. Nussbaumer, W. Niederberger, and A. Wood. Intravenous cyclosporine kinetics in renal failure.Clin. Pharmacol. Ther. 34:638–643 (1983).
F. Bozkurt, H. Stierle, P. Schollmeyer, and E. Keller. Single-dose response kinetics of cyclosporine.Clin. Nephrol. 28:10–14 (1987).
F. C. Henny, C. H. Kleinbloesem, A. J. Moolenaar, L. C. Paul, D. D. Breimer, and L. A. Van Es. Pharmacokinetics and nephrotoxicity of cyclosporine in renal transplant recipients.Transplantation 40:261–265 (1985).
A. Lindholm, S. Henricsson, M. Lind, and R. Dahlqvist. Intraindividual variability in the relative systemic availability of cyclosporin after oral dosing.Eur. J. Clin. Pharmacol. 34:1–4 (1988).
G. C. Yee, M. S. Kennedy, R. Storb, and E. D. Thomas. Pharmacokinetics of intravenous cyclosporine in bone marrow transplant patients.Transplantation 38:511–513 (1984).
B. Legg, S. K. Gupta, M. Rowland, R. W. G. Johnson, and L. R. Solomon. Cyclosporin: Pharmacokinetics and detailed studies of plasma and erythrocyte binding during intravenous and oral administration.Eur. J. Clin. Pharmacol. 34:451–460 (1988).
S. Robson, J. Neuberger, H. P. Keller, E. Abisch, W. Neiderberger, B. Von Graffenried, and R. Williams. Pharmacokinetic study of cylosporin A (Sandimmun®) in patients with primary biliary cirrhosis.Br. J. Clin. Pharmacol. 18:627–631 (1984).
W. Niederberger, M. Lemaire, G. Maurer, K. Nussbaumer, and O. Wagner. Distribution and binding of cyclosprine in blood and tissues.Transplant. Proc. 15:2419–2421 (1983).
G. J. Burckart, R. Venkataramanan, R. J. Ptachcinski, T. E. Starzl, J. C. Gartner, B. J. Zitelli, J. J. Malatack, B. W. Shaw, S. Iwatsuki, and D. H. Van Thiel. Cyclosporine absorption following orthotopic liver transplantation.J. Clin. Pharmacol. 26:647–651 (1986).
G. Burckart, T. Starzl, L. Williams, A. Sanghvi, C. Gartner, R. Venkataramanan, B. Zitelli, J. Malatack, A. Urback, W. Diven, R. Ptachcinski, B. Shaw, and S. Iwatsuki, Cyclosporine monitoring and pharmacokinetics in pediatric liver transplant patients.Transplant. Proc. 17:1172–1175 (1985).
A. J. Wood and M. Lemaire. Pharmacologic aspects of cyclosporine therapy: Pharmacokinetics.Transplant. Proc. 17:27–32 (1985).
B. D. Tarr and S. H. Yalkowsky. Enhanced intestinal absorption of cyclosporine in rats through the reduction of emulsion droplet size.Pharm. Res. 6:40–43 (1989).
M. Rowland. Critique of cyclosporine pharmacokinetic measurements.Transplant. Proc. 18:257–262 (1986).
B. Legg and M. Rowland. Saturable binding of cyclosporin A to erythrocytes: Estimation of binding parameters in renal transplant patients and implications for bioavailability assessment.Pharm. Res. 5:80–85 (1988).
M. Rowland. Critique of cyclosporine pharmacokinetic measurements.Transplant. Proc. 18:257–262 (1986).
S. D. Hall, C. B. McAllister, and G. R. Wilkinson. The assessment of bioavailability in the presence of nonlinear elimination. First-pass effect: Non-linear concept comprising an explicit solution of integrated Michaelis-Menten equations.J. Pharmacokin. Biopharm. 16:263–278 (1988).
M. O. Karlsson and U. Bredberg. Bioavailability estimated by semisimultaneous drug administration: A Monte Carlo simulation study.J. Pharmacokin. Biopharm. 18:103–120 (1990).
M. O. Karlsson and U. Bredberg. Estimation of bioavailability at a single occasion after semi-simultaneous drug administration.Pharm. Res. 6:817–821 (1989).
A. M. Mood, F. A. Graybill, and D. C. Boes.Introduction to the Theory of Statistics, 3rd ed., McGraw-Hill, Tokyo, 1974.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Karlsson, M.O., Lindberg-Freijs, A. Comparison of methods to calculate cyclosporine a bioavailability from consecutive oral and intravenous doses. Journal of Pharmacokinetics and Biopharmaceutics 18, 293–311 (1990). https://doi.org/10.1007/BF01062270
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF01062270