Abstract
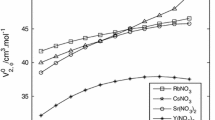
The near-infrared spectra (9500 to 11000 cm−1) of pure water and aqueous solutions of alkali halides, MgCl2, NaClO4, and R4NBr were measured at temperatures between 10 and 55°C and pressures up to 500 MPa. From the analysis of the absorption spectra the following conclusions are drawn. (1) The ice I-like open structure is destroyed and the packed structure is formed as the pressure is increased. (2) The open structure of water is destroyed by the addition of alkali halides and MgCl2 and water molecules are restricted around the ions by ion-dipole interactions. This results in a loosening of the O−H bond. (3) The perchlorate ion destroys the open structure of water and the ion-dipole interaction with water is insignificant. (4) The Bu4N+ ion forms water structure around the ion similar to that of the clathrate open structure.
Similar content being viewed by others
References
G. E. Walrafen,J. Solution Chem. 2, 159 (1973).
E. U. Franck and K. Roth,Disc. Faraday Soc. 29, 108 (1967).
V. M. Valyashko and M. Buback, and E. U. Franck,Z. Naturforsch. 35a, 549 (1980).
V. M. Valyashko, M. Buback, and E. U. Franck,Z. Naturforsch. 36a, 1169 (1981).
K. Suzuki, Y. Taniguchi, and H. Tsuchiya, inHigh Pressure Science and Technology K. D. Timmerhaus and M. S. Barber, eds., (Plenum, New York and London, 1979) p. 548.
K. Buijs and G. R. Choppin,J. Chem. Phys. 39, 2035 (1963).
K. Suzuki and M. Tsuchiya,Bull. Chem. Soc. Jpn. 48, 1701 (1975).
K. E. Bett and J. B. Cappi,Nature 207, 620 (1965).
J. W. Linowsky, Nan-I Liu, and J. Jonas,J. Chem. Phys. 65, 3383 (1976).
T. DeFries and J. Jonas,J. Chem. Phys. 66, 896 (1977).
L. A. Woolf,J. Chem. Soc. Faraday I 71, 784 (1975).
V. Vand and W. A. Senior,J. Chem. Phys. 43, 1878 (1965).
V. Fornés and J. Chaussidon,J. Chem. Phys. 68, 4667 (1978).
P. R. Philip and C. Jolicoeur,J. Phys. Chem. 77, 3071 (1973).
K. W. Bunzl,J. Phys. Chem. 71, 1358 (1967).
J. D. Worley and I. M. Klotz,J. Chem. Phys. 45, 2868 (1966).
O. D. Bonner and G. B. Woolsey,J. Phys. Chem. 72, 899 (1968).
W. C. McCabe, S. Subramanian, and H. F. Fisher,J. Phys. Chem. 74, 4360 (1970).
J. Paquette and C. Jolicoeur,J. Solution Chem. 6, 403 (1977).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Inoue, A., Kojima, K., Taniguchi, Y. et al. Near-infrared spectra of water and aqueous electrolyte solutions at high pressures. J Solution Chem 13, 811–823 (1984). https://doi.org/10.1007/BF00647696
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00647696