Abstract
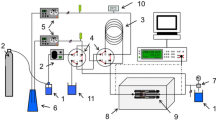
The limiting conductivity of Dy3+(aq) has been determined for the first time by linear extrapolation of conductivity measured in dilute aqueous solutions of (DyCl3+HCl) at 25°C as λo(Dy3+, aq) = (62.9±0.7) S-cm2-eq-1. A second set of conductivity measurements in dilute aqueous solutions of DyCl3 has given evidence of very slight hydrolysis of the cation, with a first hydrolysis constant of 6 x 10−8 mol-dm−3 (pK=7.2±0.5) calculated by applying the Onsager-Kim law of electrolyte mixtures.
Similar content being viewed by others
References
F. David and B. Fourest, IPNO-DRE 88-27, CNRS, Université Paris-Sud, 91406 Orsay, France.
F. David, IPNO-DRE 90-23, CNRS, Université Paris-Sud, 91406 Orsay, France.
B. Fourest, J. Duplessis, and F. David,J. Less Common Metals 92, 17 (1983);
,Radiochim. Acta 36, 191 (1984).
R. Lundqvist, E. K. Hulet, and P. A. Baisden,Acta Chem. Scand. A35, 653 (1981).
B. Fourest, J. Duplessis, and F. David,Radiochim. Acta. 46, 131 (1989).
B. Fourest, F. David, and E. Haltier,Lanth. Actinide Res. 2, 393 (1988).
J. M'Halla, M. Chemla, R. Bury, and F. David,J. Chim. Physique 85, 121 (1988).
F. H. Spedding, P. E. Porter, and J. M. Wright,J. Am. Chem. Soc. 74, 2055 (1952).
J. M'Halla and F. David,Bull. Soc. Chim. France 3–4, I-85 (1984).
F. David, B. Fourest, and J. Duplessis,J. Nucl. Mater. 130, 273 (1985).
T. Shedlovsky,J. Am. Chem. Soc. 63, 2811 (1941).
R. H. Doremus, inIon exchange, Vol. 2 J. A. Marinsky, ed., (Marcel Dekker, New York, 1969) Chap. 1.
J. E. Lind, Jr., J. J. Zwolenik, and R. M. Fuoss,J. Am. Chem. Soc. 81, 1557 (1959).
L. Onsager and S. K. Kim,J. Phys. Chem. 61, 215 (1957).
V. N. Kumok and V. V. Serebrennikov,Russ. J. Inorg. Chem. 10, 1095 (1965).
C. F. Baes, Jr. and R. E. Mesmer,The Hydrolysis of Cations (Wiley, New York, 1976).
R. Guillaumont, B. Desire, and M. Galin,Radiochem. Radioanal. Lett. 8, 189 (1971).
R. A. Robinson and R. H. Stokes,Electrolyte Solutions, 2nd edn., (Butterworth, New York, 1959) p. 143.
F. David and J. M'Halla, unpublished results.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Fourest, B., Bury, R., Morss, L.R. et al. Conductometric studies of DyCl3 in dilute aqueous solution. J Solution Chem 22, 151–162 (1993). https://doi.org/10.1007/BF00650681
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00650681