Abstract
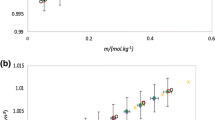
The electromotive force of the amalgam cell {NaxHg1-x‖NaCl(m)‖AgCl‖Ag} has been measured at 25°C as a function of the mole fraction x of Na in amalgams and of the molality m of NaCl in (ethylene glycol + water), (acetonitrile + water), and (1,4-dioxane + water) mixed solvents, containing up to 0.8 mass fraction of the organic component, with relative permittivities ε ≥ 27. The relevant standard molal electromotive forces \(E_{\text{m}}^ \circ \) have been determined, together with the mean molal activity coefficient γ± of NaCl as a function of its molality. In the case of (ethylene glycol + water) mixtures, a linear dependence of \(E_{\text{m}}^ \circ \) on the mass fraction of ethylene glycol is observed, which is quite unusual, The Debye-Hückel equation is applicable successfully over the whole range of molalities explored, which extends to the vicinity of the solubility limit of NaCl in each solvent. The dependence of the standard emf on the logarithm of the volume fraction of water in the aqueous-organic solvent mixtures have been analyzed in terms of Feakins and French's theory, leading to a primary hydration number 7.2 for NaCl, in good agreement with previous results employing different methods.
Similar content being viewed by others
REFERENCES
T. Mussini and F. Mazza, Electrochim. Acta, 32, 855 (1987).
D. Feakins and C. M. French, J. Chem. Soc. 663 (1957).
D. J. G. Ives and G. J. Janz, Reference Electrodes—Theory and Practice, (Academic Press, New York, 1961) (pp. 191–14).
A. Basili, P. R. Mussini, T. Mussini, and S. Rondinini, J. Chem. Thermodyn. 28, 923 (1996).
A. Basili, P. R. Mussini, T. Mussini, and S. Rondinini, Ber. Bunsenges. Phys. Chem., 101, 842 (1997).
G. J. Janz, Reference Electrodes—Theory and Practice, (Academic Press, New York, 1961) Ref. 3}, pp. 203–207.
T. Mussini, P. Longhi, and S. Rondinini, Pure Appl. Chem. 57, 169 (1985), and literature cited therein.
T. Mussini and A. Pagella, J. Chem. Eng. Data 16, 49 (1971).
E. Hückel, Physik. Z. 26, 93 (1925).
D. I. Hitchcock, J. Am. Chem. Soc. 50, 2076 (1928).
R. A. Robinson and R. H. Stokes, Electrolyte Solutions, 2nd rev. edn., (Butterworths, London, 1965), pp. 457, 458.
B. B. Owen, J. Am. Chem. Soc. 54, 1758 (1932).
M. Born, Z. Physik 1, 45 (1920).
T. Mussini, P. Longhi, and P. Giammario, Chim. Ind. (Milan) 54, 3 (1972).
T. Mussini and P. Longhi, Chim. Ind. (Milan) 54, 1093 (1972).
P. R. Mussini, T. Mussini, A. Perelli, and S. Rondinini, J. Chem. Thermodyn. 25, 1055 (1993).
W. J. Hamer and Y. C. Wu, J. Phys. Chem. Ref. Data, 1, 1047 (1972).
J. O'M. Bockris and A. K. N. Reddy, Modern Electrochemistry, Vol. 1, (Plenum Press, New York, 1970) p. 131.
A. M. Azzam, Thesis, p. 441, London, 1949, Z. Elektrochem. 58, 889 (1954).
R. Cavaliere, P. Longhi, T. Mussini, and S. Negha. Gazz. Chim. Ital. 109, 495 (1979).
P. Longhi, P. R. Mussini, S. Rondinini, and G. Tiella, Ann. Chim. (Rome), 78, 309 (1988).
P. R. Mussini, T. Mussini, and S. Rondinini, Ann. Chim. (Rome), 78, 299 (1988).
T. Mussini, P. Longhi, S. Rondinini, M. Tettamanti, and A. K. Covington, Anal. Chim. Acta, 174, 331 (1985).
S. Rondinini, C. Confalonieri, P. Longhi, and T. Mussini, Electrochim. Acta. 30, 981 (1985).
F. E. Critchfielfd, J. A. Gibson, and J. L. Hall, J. Am. Chem. Soc., 75, 1991 (1953).
T. Mussini, A. K. Covington, M. Cicognini, P. Longhi, and S. Rondinini, Ann. Chim. (Rome), 72, 639 (1982); G. Akerlöf, and O. A. Short, J. Amer. Chem. Soc. 58, 1241 (1936); F. Hovorka, R. A. Schaefer, and D. Dreisbach, J. Amer. Chem. Soc., 58, 2264 (1936); N. C. Das and P. B. Das, J. Chem. Soc. Faraday Trans. I, 74, 22 (1978); H. S. Harned and C. Calmon, J. Amer. Chem. Soc. 60, 334 (1938); G. Pistoia, Ric. Sci., 37, 731 (1967); M. Goffredi and R. Triolo, Ric. Sci., 37, 1137 (1967); F. Accascina and A. D'Aprano, Gazz. Chim. Ital. (Rome), 95, 1420 (1965); J. C. Justice, R. Bury, and C. Treiner, J. Chim. Phys. 65, 1708 (1968).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ceccattini, P.D., Mussini, P.R. & Mussini, T. Thermodynamics of NaCl in Aqueous Ethylene Glycol, Acetonitrile, and 1,4-Dioxane Mixtures from Emf Measurements at 25°C. Journal of Solution Chemistry 26, 1169–1186 (1997). https://doi.org/10.1023/A:1022981107236
Issue Date:
DOI: https://doi.org/10.1023/A:1022981107236