Abstract
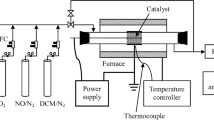
A gas-phase oxidation method using dielectric barrier discharges (DBDs) has been developed to remove SO2 and to simultaneously remove SO2 and NO from gas streams that are similar to gas streams generated by the combustion of fossil fuels. SO2 and NO removal efficiencies are evaluated as a function of applied voltage, temperature, and concentrations of SO2, NO, H2O(g), and NH3. With constant H2O(g) concentration, both SO2 and NO removal efficiencies increase with increasing temperature from 100 to 160°C. At 160°C with 15% by volume H20(g), more than 95% of the NO and 32% of the S02 are simultaneously removed from the gas stream. Injection of NH3 into the gas stream caused an increase in S02 removal efficiency to essentially 100%. These results indicate that DBD plasmas have the potential to simultaneously remove SO2 and NO from gas streams generated by large-scale fossil fuel combustors.
Similar content being viewed by others
References
J. D. Spengler, M. Brauer, and P. Koutrakis,Environ. Sci. Technol. 24, 946 (1990).
P. J. Temple,J. Air Pollut. Control Assoc. 22, 271 (1972).
E. O. Edney, D. C. Stiles, and J. W. Spence, A Laboratory Study to Evaluate the Impact of NOx and Oxidants on Atmospheric Corrosion of Galvanized Steel,ACS Svmp. Ser. 318, 172 (1986).
E. B. Cowling,Environ. Sci. Technol. 16, 110A (1982).
M. E. Bassett and J. H. Seinfeld,Atmos. Environ. 18, 1163 (1984).
C. S. Sloane and G. T. Wolff,Atmos. Environ. 19, 669 (1985).
H. B. Singh,Environ. Sci. Technol. 21, 320 (1987).
C. V. Mathai,J. Air Waste Manage. Assoc. 40, 1486 (1990).
L. M. Thomas, USEPA J. (Oct. 1987).
S. Jordan,Radial. Phys. Chem. 31, 21 (1988).
S. Machi, O. Tokunaga, K. Nishimura, S. Hashimoto, W. Kawakami, and M. Washino,Radial. Phys. Chem. 9, 371 (1977).
O. Tokunaga, K. Nishimura, and M. Washino,International Journal of Applied Radiation and Isotopes 29, 87 (1978).
N. Frank, S. Hirano, and K. Kawamura,Radial. Phys. Chem. 31, 57 (1988).
H. R. Paur, S. Jordan, and W. Baumann,J. Aerosol Sci. 19, 1397–1400 (1988).
B. Eliasson and B. Gellert,J. Appl. Phys. 68, 2026 (1990).
J. G. Calvert and W. R. Stockwell,Mechanism and Rates of the Gas-Phase Oxidations of Sulfur Dioxide and Nitrogen Oxides in the Atmosphere, Ann Arbor Scientific Publication, Ann Arbor (1984).
F. Yin, D. Grosjean, and J. H. Seinfeld,J. Geophy. Res. 91, 14417 (1986).
J. D. Butler,Air Pollution Chemistry, Academic Press, New York (1979), p. 275.
D. L. Baulch, R. A. Cox, and P. J. Crutzen,J. Phys. Chem. Ref. Data 11, 327 (1982).
O. Tokunaga and N. Suzuki,Radial. Phys. Chem. 24, 145 (1984).
C. Willis and A. D. Boyd,Int. J. Radial. Phys. Chem. 8, 71 (1976).
U. Kogelschatz, B. Eliasson, and M. Hirth,Ozone Sci. Technol. 10, 367 (1988).
B. Eliasson, M. Hirth, and U. Kogelschatz,J. Phys. D: Appl. Phys. 20, 1421 (1987).
W. R. Browne and E. E. Stone, Sulfur Dioxide Conversion under Corona Discharge Catalysis, prepared for HEW under contract PH 86-65-2 (1965).
I. Sardja and S. K. Dhali,Appl. Phys. Lett. 56, 21 (1990).
M. B. Chang, J. H. Balbach, M. J. Rood, and M. J. Kushner,J. Appl. Phys. 69, 4409 (1991).
W. C. Neely, E. I. Newhouse, and S. Pathirana,Chem. Phys. Lett. 155, 381 (1989).
J. W. Bozzelli and R. B. Barat,Plasma Chem. Plasma Process.8, 293 (1988).
G. H. Ramsey, N. Plaks, W. H. Ponder, B. E. Daniel, and L. E. Hamel, Proceedings of the 83rd Annual Air and Waste Management Association Meeting, Pittsburgh, Pennsylvania, Paper No. 90–109.2 (1990).
M. B. Chang, M. J. Kushner, and M. J. Rood,Environ. Sci. Technol. 26, 777–781 (1992).
A. M. Winer, J. W. Peters, J. P. Smith, and J. N. Pitts, Jr.,Atmos. Environ. 18, 5 (1984).
J. C. Person and D. O. Ham,Radial. Phys. Chem. 31, 1 (1988).
H. Bai, P. Biswas, and T. C. Keener, Proceedings of the 84th Annual Air and Waste Management Association Meeting, Vancouver, B.C., Canada, Paper No. 91–103.24 (1991).
R. Landreth, R. G. de Pena, and J. Heicklen,J. Phys. Chem. 89, 9 (1985).
R. W. Tock, K. C. Hoover, and G. J. Faust, SO2 Removal by Transformation to Solid Crystals of NH3 Complexes,AIChE Symp. Ser. 188, 75 (1979).
T. C. Keener and W. T. Davis, Demonstration of a Ca(OH)2/NH3 Based System for the Removal of SO2 on High Sulfur Coals, Ohio Coal Development Office, Columbus, Ohio, August, 1988.
C. C. Shale, Ammonia Injection: A Route to Clean Stacks,Pollution Control and Energy Needs, R. M. Jimeson and R. S. Spindt, eds., Advances in Chemistry Series,127, 195–205 (1973).
J. J. Carlins,Environ. Prog. 1, 2, 113–118 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chang, M.B., Kushner, M.J. & Rood, M.J. Removal of SO2 and the simultaneous removal of SO2 and NO from simulated flue gas streams using dielectric barrier discharge plasmas. Plasma Chem Plasma Process 12, 565–580 (1992). https://doi.org/10.1007/BF01447259
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF01447259