Abstract
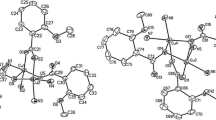
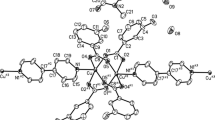
Four copper(II) complexes of betaines, [Cu2(BET)4Cl2][Cu(BET)2Cl2]Cl2 (2), [Cu2(pyBET)4Cl2]3[CuCl4]2Cl2 (3), [Cu, (pyBET)4 (H2O)2] (NO3)4 · 2H2O (4), and [Cu2(ppBET)4(H2O)2](ClO4)4 · 4H2O (5), (BET = Me3N+CH2COO−; pyBET = C5H5N+CH2COO−; ppBET=C5H5N+CH2CH2COO−), have been prepared and characterized by X-ray crystallography. These complexes all contain dimeric [Cu2 (carboxylato-O,O′)4L2] structures [basal Cu-O=1.955(4) ∼ 1.991(2), Cu ⋯ Cu=2.602(1) ∼ 2.759(1) Å] with the apical ligand L=Cl− in (2) and (3) [Cu-Cl=2.415(1) ∼ 2.436(3) Å] and L = H2O in (4) and (5) [Cu-OH2=2.158(4) ∼ 2.192(3) Å]; also present are a discrete [Cu(BET)2Cl2] molecule with a compressed tetrahedral CuO2Cl2 chromophore involving two unidentate carboxylate ligands [Cu-O=1.916(2), Cu-Cl=2.254(1) Å] in (2), and a discrete C3v [CuCl4]2− anion in (3). Generally the intradimer Cu ⋯ Cu distance may be correlated to the electronic repulsion of the metal-ligand bonds in the CuO4L chromophore, as well as the steric interaction between the carboxylate moieties and the apical ligand.
Similar content being viewed by others
References
van Niekerk, J. N.; Schoening, F. R. L.Acta Crystallogr. 1953, 6, 227;
Meester, P.; Fletcher, S. R.; Skapski, A. G.J. Chem. Soc, Dalton Trans. 1973, 2575.
Doedens, R.J. Prog. Inorg. Chem. 1976,21, 209.
Carerick, J.; Thornton, P.Adv. Inorg. Chem. 1977,21, 291.
Melnik, M.Coord. Chem. Rev. 1982,42, 259.
Steward, O. W.; McAfee, R. C.; Chang, S.-C; Pisker, S. R.; Schreiber, W. J.; Jurry, C. F.; Taylor, C. E.; Pletcher, J. F.; Chen, C.-S.Inorg. Chem. 1986,25, 771.
Porter, L. C.; Dickman, M. H.; Dordens, R.J. Inorg. Chem. 1986,25, 678.
Goodgame, D. M. L.; Hill, N. J.; Marsham, D. F.; Skapski, A. C.Chem. Commun. 1969, 629.
Borel, M. M.; Leclaire, A.Acta Crystallogr., Sect. B,1976,32, 1275.
Moreland, J. A.; Doedens, R.J. J. Am. Chem. Soc. 1975,97, 508.
Figgis, B. N.; Martin, R. L.J. Chem. Soc. 1956, 3837.
Forster, L. S.; Ballhausen, C.J. Acta Chem. Scand. 1962,16, 1385;
Boudreaux, E. A.Inorg. Chem. 1964,3, 506.
Kato, M.Coord. Chem. Rev. 1988, 92, 45;
Asakawa, T.; Inoue, M.; Hara, K.; Kubo, M.Bull. Chem. Soc. Jpn. 1972,45, 1054;
Zelonka, R. A.; Baird, M. C.;Inorg. Chem. 1972,11, 134;
Beddoes, R. L.; Connor, J. A.; Dubowski, D.; Jones, A. C; Mills O. S.; Price, R.J. Chem. Soc, Dalton Trans. 1979, 781.
Hay, P. J.; Thibeault, J. C.; Hoffman, R.J. Am. Chem. Soc. 1975,97, 4884;
Harcourt, R. D.; Skrezenek, F. L.; Maclagan, R. G. A. R.J. Am. Chem. Soc. 1986,108, 5403.
Rao, V. M.; Sathyanarayana, D. N.; Manohar, H.J. Chem. Soc., Dalton Trans. 1983, 2167.
Chen, X.-M.; Mak, T. C. W.Acta Crystallogr., Sect. C. 1992,48, 1211.
Chen, X.-M.; Mak, T. C. W.J. Cryst. Spectrosc. Res. 1991,21, 27;
J. Chem. Soc, Dalton Trans. 1991, 1219;
Polyhedron 1991,10, 1723.
Chen, X.-M.; Mak, T. C. W.Inorg. Chem. Acta. 1991,182, 139;
J. Chem. Soc., Dalton Trans. 1991, 3253;
Struct. Chem. 1992,3, 369;
Mak, T. C. W.; Chen, X.-M.Aust. J. Chem. 1991,44, 639;
Huang, W.-Y.; Lü, L.; Chen, X.-M.; Mak, T. C. W.Polyhedron 1991,10, 2687.
Chen, X.-M.; Mak, T. C. W.;J. Chem. Soc., Dalton Trans. 1992, 1585.
Sparks, R. A. InCrystallographic Computing Techniques; Ahmed, F. R., Ed.; Munksqaard: Copenhagen, 1976; pp. 452–467.
Diamond, R.Acta Crystallogr., Sect. A. 1969, 27, 43.
Kopfmann, G.; Huber, R.Acta Crystallogr., Sect. A. 1968,24, 348.
Sheldrick, G. M. inComputational Crystallography; Sayre, D., Ed.; Oxford University Press: New York, 1982; pp. 506–516;
Sheldrick, G. M. InCrystallographic Computing. 3: Data Collection, Structure Determination, Proteins, and Databases; Sheldrick, G. M.; Krüger, C; Goddard, R., Eds.; Oxford University Press: New York, 1985; pp. 175–189.
International Tables for X-ray Crystallography, Vol. IV; Ibers, J. A.; Hamilton, W. C., Eds.; Kynoch Press: Birmingham, U.K., 1974; pp 55, 99, 149. (Now distributed by Kluwer Academic Publishers: Dordrecht, The Netherlands).
Hone, H.; Husebye, S.; Kato, M.; Meyers, E. A.; Muto, Y.; Tokii, T.; Zingaro, R. A.Acta Chem. Scand. A 1986,40, 579.
Willett, R. D.Coord. Chem. Rev. 1991,109, 181;
Smith, D. W.Coord. Chem. Rev. 1976,21, 93;
Halvrorson, K. H.; Patterson, C; Willet, R. D.Acta Crystatlogr., Sect. B. 1990,46, 508.
Hathaway, B. J. InComprehensive Coordination Chemistry; Wilkinson, G.; Gillard, R. D.; McClerverty, J.A., Eds.; Pergamon Press: Oxford, 1987; Vol. 5, Chap. 54, p 606;
Mehrotra, R. C.; Bohra, R.Metal Carboxylates; Academic Press: New York, 1983; Chap. 4, pp 286–295.
Bertrand, J. A.; Kalyanranman, A. R.Inorg. Chim. Acta 1971,5, 341.
Clay, R. M.; Murray-Rust, P.; Murray-Rust, J.J. Chem. Soc.,Dalton Trans. 1973, 595.
Harrison, W.; Rettig, S.; Trotter, J.J. Chem. Soc. A 1972, 1852.
Simonov, Yu. A.; Ivanov, V. I.; Ablov, A. V.; Milkova, L. N.Zh. Struct. Khim. 1976,17, 516;
Tarkahova, T. N.; Ablov, A. V.Kristallografiya 1969,13, 611.
Morosin, B.; Hughes, R. C.; Soos, Z. G.Acta Crystallogr., Sect. B 1975,31, 762.
Dovey, G.; Stephens, F. S.J. Chem. Soc. A 1970, 1626;
Kettle, S. F. A.; Pioli, A. J. P.J. Chem. Soc. A. 1968, 1242.
Simonov, Yu. A.; Milkova, L. N.; Ablov, A. V.; Malkinovskii, T. I.Dokl. Akad. Nauk. USSR 1976,229, 1134.
Ivanov, V. I.; Simonov, Yu. A.; Ablov, A. V.; Milkova, L. N.Kristallografiya 1974,19, 1286;
Yablokov, Yu. V.; Mosina, L. N.; Simonov, Yu. A.; Milkova, L. N.; Ablov, A. V.; Ivanov, V. I.Zh. Struct. Khim 1978,19, 42.
Moreland, J. A.; Doedens, R.J. Inorg. Chem. 1978,3, 674.
Pauling, L.The Nature of Chemical Bond, 3rd. ed., Cornell University Press: Ithaca, NY, 1960; p 260
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Chen, XM., Mak, T.C.W. Metal-betaine interactions. Part 17: A study of intradimer Cu · · · Cu distance variation in copper(II) betaine complexes containing [Cu2 (carboxylato-O,O′)4L2]n+ species. Struct Chem 4, 247–259 (1993). https://doi.org/10.1007/BF00673699
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00673699