Abstract
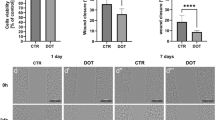
The relevance of tumor necrosis factor-α (TNF-α) inducing early inflammatory reactions in the liver after hemorrhagic shock, for example, leukocyte adhesion, has been well described. This study evaluated the anti-inflammatory effects of a monoclonal antibody against TNF-α (TN3.19.12) in terms of the time of application, namely, prior to shock induction, at the time of resuscitation, and after resuscitation. The hepatic micro-circulation was investigated by intravital fluorescence microscopy in female Sprague-Dawley rats undergoing severe hemorrhagic shock for 60 min and subsequent resuscitation. TN3.19.12 or placebo was given in a randomized and blinded manner either 60 min prior to shock induction, l min prior to resuscitation, or 15 min after the onset of resuscitation. The number of firmly adherent leukocytes in the livers of treated animals depended on the time of application of TN3.19.12. Leukocyte adhesion was significantly reduced when TN3.19.12 was given prior to shock induction or at the time of resuscitation and was less effective when administered after the onset of resuscitation. The results further confirm that TNF-α initiates very early pathological leukocyte adhesion in the liver 5 h following shock. Inhibition of leukocyte adhesion after shock, however, depends strongly on the time of TNF-α blocking. While TN3.19.12 prior to shock induction resulted in most effective attenuation, only very early treatment allowed limitation of posttraumatically increased leukocyte adhesion.
Similar content being viewed by others
Abbreviations
- CO :
-
Cardiac output
- HR :
-
Heart rate
- IL :
-
Interleukin
- MAP :
-
Mean arterial pressure
- WBC :
-
White blood cells
References
Stricter RM, Kunkel SL, Bone RC (1993) Role of tumor necrosis factor-α in disease states and inflammation. Crit Care Med 21:447–463
Tracey KJ, Beutler B, Lowry SF, Merryweather J, Volpe S, Milsark IW, Hariri RJ, Fahey TJ, Zentella A, Albert JD, et al (1986) Shock and tissue injury induced by recombinant human cachectin. Science 234:470–414
Rabinovici R, John R, Esser KM, Vernick J, Feuerstein G (1993) Serum tumor necrosis factor-alpha profile in trauma patients. J Trauma 35:698–702
Jutila MA (1992) Leukocyte traffic to sites of inflammation. APMIS 100:191–201
Furie MB, McHugh DD (1989) Migration of neutrophils across endothelial monolayers is stimulated by treatment of the monolayers with interleukin-1 or tumor necrosis factor-α. J Immunol 143:3309–3317
Von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors K-E, Butcher EC (1991) Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte β2 integrins in vivo. Proc Natl Acad Sci USA 88:7538–7542
Pohlmann TH, Stannes KA, Beatty PG, Ochs HD, Harlan JM (1986) An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin-1, and tumor necrosis factor-α increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol 136:4548–4553
Pelikane JV, DeMaria EJ, Abd-Elfattah A, Reines HD, Vannice JL, Carson KW (1993) Interleukin-1 receptor antagonist improves survival and preserves organ adenosine-5′-triphosphate after hemorrhagic shock. Surgery 114:278–284
Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW (1993) Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology 17:915–923
Wherry JC, Pennington JE, Wenzel RP (1993) Tumor necrosis factor and the therapeutic potential of anti-tumor necrosis factor antibodies. Crit Care Med 21:436–440
Bauer C, Marzi I, Bauer M, Fellger H, Larsen R (1995) Interleukin-1 receptor antagonist attenuates leukocyte-endothelial interactions in the liver following hemorrhagic shock in the rat. Crit Care Med 23:1099–1105
Emerson TEJ, Lindsey DC, Jesmok GJ (1992) Efficacy of monoclonal antibody against tumor necrosis factor-a in an endotoxemic baboon model. Circ Shock 38:75–84
Marzi I, Bauer M, Secchi A, Bahrami S, Redl H, Schlag G (1995) Effect of anti-TNF-α on leukocyte adhesion in the liver after hemorrhagic shock: an intravital microscopic study in the rat. Shock 3:27–33
Schlag G, Bahrami S, Yao YM, et al (1994) Anti-TNF monoclonal antibody (mab) against haemorrhage-induced pa mortality in rats. Shock 1 [Suppl]:1 (abstract)
Hinshaw LB, Tekamp-Olson P, Chang ACK, Lee PA, Taylor FB, Murray CK, Peer GT, Emerson TE, Passey RB, Kuo GC (1990) Survival of primates in LD100 septic shock following therapy with antibody to tumor necrosis factor (TNF). Circ Shock 30:279–292
McCuskey RS, Reilly FD, McCuskey PA, Dimlich RVW (1978) In vivo microscopy of the hepatic microvascular system. Bibl Anat 18:73–76
Marzi I, Bauer C, Hower R, Bühren V (1993) Leukocyte-endothelial cell interactions in the liver after hemorrhagic shock in the rat. Circ Shock 40:105–114
Marzi I, Walcher F, Bühren V (1993) Macrophage activation and leukocyte adhesion after liver transplantation. Am J Physiol 265:G172-G177
Engelberts I, Von Asmuth EJU, Van der Linden CJ, Buurman WA (1991) The interrelation between TNF-, IL-6, and PAF secretion induced by LPS in an in vivo and in vitro murine model. Lymphokine Cytokine Res 10:127–131
Marzi I, Knee J, Bühren V, Menger MD, Trentz O (1992) Reduction by Superoxide dismutase of leukocyte-endothelial adherence after liver transplantation. Surgery 111:90–97
Rappaport AM (1963) Acinar units and the pathophysiology of the liver. In: Rouiller C (ed) The liver. Morphology, biochemistry, physiology, vol. 1. Academic, London, pp 265–328
Kreil EA, Greene E, Fitzgibbon C, Robinson DR, Zapol WM (1989) Effects of recombinant human tumor necrosis factor alpha, lymphotoxin, and Escherichia coli lipopolysaccharide on hemodynamics, lung microvascular permeability, and eicosanoid synthesis in anesthetized sheep. Circ Shock 65:502–514
Han JJ, Windsor A, Drenning DH, Leeper-Woodford S, Mullen PG, Bechard DH, Sugerman HJ, Fowler III AA (1994) Release of endothelin in relation to tumor necrosis factor-a in porcine Pseudomonas aeruginosa-induced septic shock. Shock 1:343–346
Bauer M, Zhang JX, Bauer I, Clemens MG (1994) ET-1 induced alterations of hepatic microcirculation: sinusoidal and extrasinusoidal sites of action. Am J Physiol 267:G143-G149
Goto M, Takei Y, Kawano S, Tsuji S, Masuda I, Nishimura Y, Okumura S, Kashiwagi T, Fusamoto H, Kamada T (1994) Endothelin-1 is involved in the pathogenesis of ischemia/reperfusion liver injury by hepatic microcirculatory disturbances. Hepatology 19:675–681
Kawada N, Tran-Thi T-A, Klein H, Decker K (1993) The contraction of hepatic stellate (ito) cells stimulated with vasoactive substances: possible involvement of endothelin 1 and nitric oxide in the regulation of the sinusiodal tonus. Eur J Biochem 213:815–823
Marzi I, Bauer M, Secchi A, Hower R, Larsen R, Bühren V (1992) Time course and pattern of hepatic leukocyte-endothelial interaction after hemorrhagic shock in the rat. Circ Shock 37:15
Ley K (1993) Leukocyte rolling: transient adhesion mediated by vascular selectins. Eur J Physiol Suppl:R8
Steinhoff G, Behrend M, Schrader B, Duijvestijn AM, Wonigeit K (1993) Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia. Am J Pathol 142:481–488
Vollmar B, Glasz J, Menger MD, Messmer K (1994) Reduktion des postischämischen Reperfusionsschadens der Leber durch anti-ICAM-1. Chirurgisches Forum 1994 für experimentelle und klinische Forschung, pp 17–20
Luscinskas FW, Cybulsky MI, Kiely J-M, Peckins CS, Davis VM, Gimbrone MA Jr (1991) Cytokine-activated human endothelial monolayers support enhanced neutrophil transmigration via a mechanism involving both endothelial-leukocyte adhesion molecule-1 and intercellular adhesion molecule-1. J Immunol 146:1617–1625
Kishimoto TK, Jutila MA, Berg EL, Butcher EC (1989) Neutrophil Mac-1 and MEL-14 adhesion proteins inversely regulated by chemotactic factors. Science 245:1238–1241
Pohlman TH, Stanness KA, Beatty PG, Ochs HD, Harlan JM (1986) An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukinl, and tumor necrosis factor-a increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol 136:4548–4553
Bouwens L, Baekeland M, de Zanger R, Wisse E (1986) Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology 6:718–722
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bauer, C., Roth, W., Bahrami, S. et al. Attenuation of shock-induced inflammation in the rat liver depends on the time of TNF-α inhibition. J Mol Med 74, 51–58 (1996). https://doi.org/10.1007/BF00202072
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00202072