Abstract
Objective
To determine the magnitude and time course of adrenergic effects on metabolism in volunteers and possible implications for the use of sympathomimetics in the critically ill.
Design
Descriptive laboratory investigation.
Subjects
7 volunteers.
Intervention
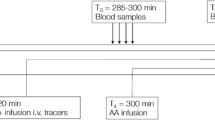
Primed continuous infusions of stable isotope tracers ([15N2]-urea, [6,6-D2]-glucose, [methyl-D3]-L-leucine, [15N]-L-alanine) were used. After isotopic steady state had been reached an infusion of adrenaline (0.1 μg/kg/min) was administered (4 h). Isotopic enrichment was measured using gas chromatography-mass spectrometry and the corresponding rates of appearance were calculated.
Measurements and main results
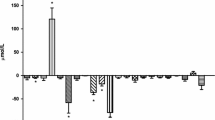
Glucose production increased from 14.1±1.2 to 21.5±2.0 μmol/kg/min (p<0.05) after 80 min of adrenergic stimulation and then decreased again to 17.9±1.2 μmol/kg/min after 240 min. Leucine and ketoisocaproate (KIC) fluxes were 2.3±0.2 and 2.6±0.2 μmol/kg/min, respectively, at baseline and gradually decreased to 1.8±0.2 and 2.2±0.1 μmol/kg/min, respectively, after 240 min of adrenaline infusion (bothp<0.05). Alanine flux increased from 3.7±0.5 to 6.9±0.9 μmol/kg/min (p<0.05) after 80 min of adrenergic stimulation. Urea production slightly decreased from 4.8±0.9 to 4.3±0.8 μmol/kg/min during adrenaline (p<0.05).
Conclusions
Adrenaline induced an increase in glucose production lasting for longer than 240 min. The decrease in leucine and KIC flux suggests a reduction in proteolysis, which was supported by the decrease in urea production. The increase in alanine flux is therefore most likely due to an increase in de-novo synthesis. The ammonia donor for alanine synthesis in peripheral tissues and the target for ammonia after alanine deamination in the liver remain to be investigated. These results indicate that adrenaline infusion most probably will not promote already enhanced proteolysis in critically ill patients. Gluconeogenesis is an energy consuming process and an increase may deteriorate hepatic oxygen balance in patients.
Similar content being viewed by others
References
Cryer PE (1980) Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N Engl J Med 303:436–444
Katzeff HL, O'Connell M, Horton ES, Danforth E, Young JB, Landsberg L (1986) Metabolic studies in human obesity during overnutrition and undernutrition: thermogenic and hormonal response to norepinephrine. Metabolism 35:166–175
Ensinger H, Stein B, Jäger O, Grünert A, Ahnefeld FW (1992) Relationship between infusion rates, plasma concentrations, cardiovascular and metabolic effects during infusion of norepinephrine in healthy volunteers. Crit Care Med 20:1250–1256
Ensinger H, Lindner KH, Dirks B, Kilian J, Grünert A, Ahnefeld FW (1992) Adrenaline: relationship between infusion rate, plasma concentration, metabolic and haemodynamic effects in volunteers. Eur J Anaesthesiol 9:435–446
Clutter WE, Bier DM, Shah SD, Cryer PE (1980) Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest 66:94–101
Rizza RA, Haymond MW, Cryer PE, Gerich JE (1979) Differential effects of epinephrine on glucose production and disposal in man. Am J Physiol 237:E356-E362
Del Prato S, DeFronzo RA, Castellino P, Wahren J, Alvestrand A (1990) Regulation of amino acid metabolism by epinephrine. Am J Physiol 258:E878-E887
Miles JM, Nissen SL, Gerich JE, Haymond MW (1984) Effects of epinephrine infusion on leucine and alanine kinetics in humans. Am J Physiol 247:E166-E172
Felig P (1973) Progress in endocrinology and metabolism. The glucose-alanine cycle. Metabolism 22:179–207
Castellino P, Luzi L, Del Prato S, DeFronzo R (1990) Dissociation of the effects of epinephrine and insulin on glucose and protein metabolism: Am J Physiol 258:E117-E125
Matthews DE, Pesola G, Campbell RG (1990) Effect of epinephrine on amino acid and energy metabolism in humans. Am J Physiol 258:E948-E956
Dirks B, Vorwalter C, Grünert A, Ahnefeld FW (1988) Basal plasma-catecholamine-level determination using HPLC-ED and different sample cleanup techniques. Chromatographia 25:223–229
Jauch KW, Hartl WH, Georgieff M, Wolfe RR, Dietze GJ, Günther B (1988) Low-dose bradykinin infusion reduces endogenous glucose production in surgical patients. Metabolism 37:185–190
Wolfe RR (1984) Tracers in metabolic research. Liss, New York
Sacca L, Morrone G, Cicala M, Corso G, Ungaro B (1980) Influence of epinephrine, norepinephrine, and isoproterenol on glucose homeostatis in normal man. J Clin Endocrinol Metab 50:680–684
Sacca L, Vigorito C, Cicala M, Corso G, Sherwin RS (1983) Role of gluconeogenesis in epinephrine-stimulated hepatic glucose production in humans. Am J Physiol 245:E294-E302
Nilsson LH, Fürst P, Hultman E (1973) Carbohydrate metabolism of the liver in normal man under varying dietary conditions. Scand J Clin Lab Invest 32:331–337
Consoli A, Kennedy F, Miles J, Gerich J (1987) Determination of Krebs cycle metabolic carbon exchange in vivo and its use to estimate the individual contributions of gluconeogenesis and glycogenolysis to overall glucose output in man. J Clin Invest 80: 1303–1310
Stevenson RW, Steiner KE, Connolly CC, Fuchs H, Alberti KGMM, Williams PE, Cherrington AD (1991) Dose-related effects of epinephrine on glucose production in conscious dogs. Am J Physiol 260:E363-E370
Kusaka M, Ui M (1977) Activation of the Cori cycle by epinephrine. Am J Physiol 232:E145-E155
Porte D (1967) A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest 46:86–94
Adibi SA (1976) Metabolism of branched-chain amino acids in altered nutrition. Metabolism 25:1287–1302
Matthews DW, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM (1980) Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-13C] leucine. Am J Physiol 238:E473-E479
Schwenk WF, Beaufrere B, Haymond MW (1985) Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol 249:E646-E650
Yang RD, Matthews DE, Bier DM, Young VR (1984) Alanine kinetics in humans: influence of different isotopic tracers. Am J Physiol 247:E634-E638
Haymond MW, Miles JM (1982) Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes 31:86–89
Ben-Galim E, Hruska K, Bier DM, Matthews DE, Haymond MW (1980) Branched-chain amino acid nitrogen transfer to alanine in vivo in dogs. J Clin Invest 66:1295–1304
Garber AJ, Karl IE, Kipnis DN (1976) Alanine and glutamine synthesis and release from skeletal muscle. IV. Beta-adrenergic inhibition of amino acid release. J Biol Chem 251:851–857
Felig PJ, Wahren J, Räf L (1973) Evidence of inter-organ amino acid transport by blood cells in humans. Proc Natl Acad Sci USA 70:1775–1779
Felig P (1975) Amino acid metabolism in man. Annu Rev Biochem 44:933–955
Jahoor F, Wolfe RR (1987) Reassessment of primed constant-infusion tracer method to measure urea kinetics. Am J Physiol 252: E557-E564
Jahoor F, Peters EJ, Wolfe RR (1990) The relationship between gluconeogenic substrate supply and glucose production in humans. Am J Physiol 258:E288-E296
Chochinov RH, Perlman K, Moorhouse JA (1978) Circulating alanine production and disposal in healthy subjects. Diabetes 27:287–295
Consoli A, Nurjhan N, Reilly JJ, Bier DM, Gerich JE (1990) Contribution of liver and skeletal muscle to alanine and lactate metabolism in humans. Am J Physiol 259:E667-E684
Mortimore GE, Poso AR (1987) Intracellular protein catabolism and its control during nutrient deprivation and supply. Ann Rev Nutr 7:534–564
Häussinger D (1990) Nitrogen metabolism in liver: structural and functional organization and physiologic relevance. Biochem J 267:281–290
Yang RD, Matthews DE, Bier DM, Wen ZM, Young NR (1986) Response of alanine metabolism in humans to manipulation of dietary protein and energy intakes. Am J Physiol 250:E39-E46
Jungas RL, Halperin ML, Brosnan JT (1992) Quantitative analysis of amino acid oxidation and related gluconeogenesis in humans. Physiol Rev 72:419–448
Dahn MS, Lange P, Lobdell K, Hans B, Jacobs LA, Mitchell RA (1987) Splanchnic and total body oxygen consumption differences in septic and injured patients. Surgery 101:69–80
Takala J, Ruokonen E (1992) Underlying disease modifies the response of regional blood flow to dobutamine. Crit Care Med 20 [Suppl]:114
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ensinger, H., Träger, K., Geisser, W. et al. Glucose and urea production and leucine, ketoisocaproate and alanine fluxes at supraphysiological plasma adrenaline concentrations in volunteers. Intensive Care Med 20, 113–118 (1994). https://doi.org/10.1007/BF01707665
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01707665