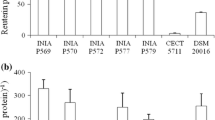
Abstract
While most strains of heterofermentative lactobacilli and strains of Leuconostoc species contained only traces of a dehydratase reacting with glycerol or propanediol-1,2, three strains of Lactobacillus brevis and one strain of L. buchneri that metabolized glycerol readily in the presence of glucose, contained propanediol-1,2 dehydratase (EC 4.2.1.28). This cobamide requiring enzyme from L. brevis B 18 was partially purified. It reacts with the substrates propanediol-1,2, glycerol and ethanediol-1,2 with the relative activities of about 3:2:1. This ratio remained unchanged throughout the purification procedure. The substrate affinities were measured: propanediol-1,2 K m=0.6 mM, glycerol K m=4 mM, ethanediol-1,2 K m=5.3 mM coenzyme B12 (substrate glycerol) K m=0.007 mM. The activity of the dehydratase was promoted by potassium or ammonium ions and inhibited by sodium, lithium, magnesium or specially manganese. The apparent molecular weight of propanediol-1,2 dehydratase was determined as Mr=180,000.
Similar content being viewed by others
References
Bachovchin WWE, Moore RG, Richards JH (1977) Mechanism of action of adenosylcobalamine: glycerol and other substrate analogues as substrates and inactivators for propanediol dehydratase-kinetics, stereospecificity and mechanism. Biochem 16:1082–1092
Caspritz G, Radler F (1983) Malolactic enzyme of Lactobacillus plantarum. J Biol Chem 258:4907–4910
Eggstein M, Kuhlmann E (1974) Triglyceride and Glycerin (alkalische Verseifung). In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, Bd II. Verlag Chemie, Weinheim/ Bergstraße
Forage RG, Foster AM (1979) Resolution of the coenzyme B-12 dependent dehydratase of Klebsiella sp. and Citrobacter freundii. Biochim Biophys Acta 569:249–258
Forage RG, Lin ECC (1982) dha system mediating aerobic and anaerobic dissimilation of glycerol in Klebsiella pneumoniae NCIB 418. J Bacteriol 151:591–599
Lee HA Jr, Abeles RH (1963) Purification and properties of dioldehydrase, an enzyme requiring a cobamide coenzyme. J Biol Chem 238:2367–2373
Linn S, Lehmann JR (1965) An endonuclease from Neurospora crassa specific for polynucleotides lacking an ordered structure. J Biol Chem 240:1287
Schneider Z, Larsen EG, Jacobson G, Pawelkiewicz J (1970) Purification and properties of glycerol dehydrase. J Biol Chem 245:3388–3396
Schütz H, Radler F (1984) Anaerobic reduction of glycerol to propanediol-1,3 by Lactobacillus brevis and Lactobacillus buchneri. Syst Appl Microbiol (in press)
Smiley KL, Sobolov M (1962) A cobamide-requiring glycerol dehydrase from an acrolein-forming Lactobacillus. Arch Biochem Biophys 97:538–543
Smiley KL, Sobolov M (1964) Some properties of the glycerol dehydrase system from a species of Lactobacillus. Ann New York Acad Sci 112:706–712
Toraya T, Fukui S (1977) Immunochemical evidence for the difference between coenzyme B-12 dependent diol dehydrase and glycerol dehydrase. Eur J Biochem 76:285–289
Toraya T, Kuno S, Fukui S (1980) Distribution of coenzyme B-12 dependent diol dehydratase and glycerol dehydratase in selected genera of Enterobacteriaceae and Propionibacteriaceae. J Bacteriol 141:1439–1442
Toraya T, Ushio K, Fukui S, Hogenkamp HPC (1977) Studies on the mechanism of the adenosylcobalamin-dependent dioldehydratase reaction by the use of analogs of the coenzyme. J Biol Chem 252:963–970
Warburg O, Christian W (1941) Isolierung und Kristallisation des Gärungsferments Enolase. Biochem 310:384–421
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. Dr. H. G. Schlegel on behalf of his 60th birthday
Rights and permissions
About this article
Cite this article
Schütz, H., Radler, F. Propanediol-1,2-dehydratase and metabolism of glycerol of Lactobacillus brevis . Arch. Microbiol. 139, 366–370 (1984). https://doi.org/10.1007/BF00408381
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00408381