Abstract
By thin layer chromatographic, gas-liquid chromatographic, and mass spectrometric methods 1,2,3,4-tetrahydroxypentane-29-hopane (THBH) was shown to occur in Zymomonas mobilis. This compound contributed up to 20% to the total lipids.
The fatty acid pattern and the content of hopanoids (hopene, hopanol, and THBH) were determined in batch and continuous cultures. In late exponential cells from batch cultures the relative amount of palmitic acid was increased partially at the expense of cis-vaccenic acid, when the initial glucose concentrations were increased. In a batch culture, THBH reached a maximum value in the early exponential growth phase.
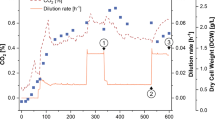
In an anaerobic continuous culture with a low glucose feed concentration, the THBH content and the relative amount of cis-vaccenic acid were low. The contribution of both compounds increased strongly with increasing glucose feed concentrations (i.e. at higher steady-state ethanol concentrations). The same result was found with aerobic continuous cultures which produced significant amounts of acetaldehyde and acetic acid, in addition to ethanol and carbon dioxide.
It was concluded that stability and permeability of the cytoplasmic membrane of the ethanol producing bacterium Z. mobilis was regulated by variations in the distribution of hopanoids and fatty acids.
Similar content being viewed by others
Abbreviations
- 14:0:
-
myristic acid
- 16:0:
-
palmitic acid
- 18:1:
-
cisvaccenic acid
- THBH:
-
1,2,3,4-tetrahydroxypentane-29-hopane
References
Andreasen AA, Stier TJB (1954) Anaerobic nutrition of Saccharomyces cerevisiae. II. Unsaturated fatty acid requirement for growth in a defined medium. J Cell Comp Physiol 43:272–282
Banauch D, Brümmer W, Ebeling W, Metz H, Rindfrey H, Lang H, Leybold K, Rick W (1975) Eine Glucose-Dehydrogenase für die Glucose-Bestimmung in Körperflüssigkeiten. Z. Klin Chem Klin Biochem 13:101–107
Barrow KD, Collins JG, Rogers PL, Smith GM (1983) Lipid composition of an ethanol-tolerant strain of Zymomonas mobilis. Biochim Biophys Acta 753:324–330
Beaven MJ, Charpentier C, Rose AH (1982) Production and tolerance of ethanol in relation to phospholipid fatty-acyl composition in Saccharomyces cerevisiae NCYC 431. J Gen Microbiol 128:1447–1455
Benz R, Hallmann D, Poralla K, Eibl H (1983) Interaction of hopanoids with phosphatidylcholines containing oleic and ω-cyclohexyldodecanoid acid in lipid bilayer membranes. Chem Phys Lipids 34:7–24
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Canad J Biochem Physiol 37:911–917
Bringer S, Finn RK, Sahm H (1984) Effect of oxygen on the metabolism of Zymomonas mobilis. Arch Microbiol 139:376–381
Carey VC, Ingram LO (1983) Lipid composition of Zymomonas mobilis: effects of ethanol and glucose. J Bacteriol 154:1291–1300
Dombek KM, Ingram LO (1984) Effects of ethanol on the Escherichia coli plasma membrane. J Bacteriol 157:233–239
Finn RK, Bringer S, Sahm H (1984) Fermentation of arabinose to ethanol by Sarcina ventriculi. Appl Microbiol Biotechnol 19:161–166
Hossack JA, Rose AH (1976) Fragility of plasma membranes in Sacchromyces cerevisiae enriched with different sterols. J Bacteriol 127:67–75
Ingram LO, Vreeland NS (1980) Differential effects of ethanol on the Escherichia coli cell envelope. J Bacteriol 144:481–488
Janssens JH, Burris N, Woodward A, Bailey RB (1983) Lipid-enhanced ethanol production by Kluyveromyces fragilis. Appl Environ Microbiol 45:598–602
Kannenberg E (1983) Membraneigenschaften von Hopanoiden und Lipiden, die Fettsäuren mit endständigen Verzweigungen und ω-Cyclohexanringen enthalten. Dissertation, Eberhard-Karls-Universität Tübingen, FRG
Kannenberg E, Blume A, Poralla K (1980) A hopanoid from the thermo-acidophilic Bacillus acidocaldarius condenses membranes. Naturwissenschaften 67:458
Langworthy TA, Mayberry WR (1976) 1,2,3,4-Tetrahydroxy-pentane-substitued pentacyclic triterpene from Bacillus acidocaldarius. Biochim Biophys Acta 431:570–577
Laudrin J, Goma G (1982) Ethanol production by Zymomonas mobilis: effect of teperature on cell growth, ethanol production and intracellular ethanol accumulation. Biotechnol Lett 4:537–542
Millar DG, Griffiths-Smith K, Algar E, Scopes RK (1982) Activity and stability of glycolytic enzymes in the presence of ethanol. Biotechnol Lett 4:601–606
Nes WR, McKean ML (1977) Biochemistry of steroids and other isopentenoids. University Park Press, Baltimore London Tokyo
Ohta K, Hayashida S (1983) Role of Tween 80 and monoolein in a lipid-sterol-protein complex which enhances ethanol tolerance of sake yeasts. Appl Environ Microbiol 46:821–825
Ohta K, Supanwong K, Hayashida S (1981) Environmental effects on ethanol tolerance of Zymomonas mobilis. J Fermen Technol 59:435–439
Ourisson G, Albrecht P, Rohmer M (1984) The microbial origin of fossil fuels. Scientific American, August, 34–41
Poralla K (1982) Considerations on the evolution of steroids as membrane components. FEMS Microbiol Lett 13:131–135
Poralla K, Härtner T, Kannenberg E (1984) Effect of temperature and pH on the hopanoid content in Bacillus acidocaldarius. FEMS Microbiol Lett 23:253–256
Poralla K, Kannenberg E, Blume A (1980) A glycolipid containing hopane isolated from the acidophilic, thermophilic Bacillus acidocaldarius, has a cholesterol-like function in membranes. FEBS Lett 113:107–110
Rogers PL, Lee KJ, Tribe DE (1979) Kinetics of alcohol production by Zymomonas mobilis at high sugar concentrations. Biotechnol Lett 1:165–170
Rohmer M, Ourisson G (1976) Structure des bactériohopanetétrols d'Acetobacter xylinum. Tetrahedron Lett 3633–3636
Ross HNM, Collins MD, Tindall BJ, Grant WD (1981) A rapid procedure for the detection of archaebacterial lipids in halophilic bacteria. J Gen Microbiol 123:75–80
Rowe ES (1983) Lipid chain length and temperature dependence of ethanol-phospatidylcholine interactions. Biochemistry 22: 3299–3305
Ryu DDY, Kim YJ, Kim JH (1984) Effect of air supplement on the performance of continuous ethanol fermentation system. Biotechnol Bioeng 26:12–16
Thomas DS, Hossack JA, Rose AH (1978) Plasma-membrane lipid composition and ethanol tolerance in Saccharomyces cerevisiae. Arch Microbiol 117:239–245
Tornabene TG, Holzer G, Bittner AS, Grohmann K (1982) Characterization of the total extractable lipids of Zymomonas mobilis var mobilis. Can J Microbiol 28:1107–1118
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bringer, S., Härtner, T., Poralla, K. et al. Influence of ethanol on the hopanoid content and the fatty acid pattern in batch and continuous cultures of Zymomonas mobilis . Arch. Microbiol. 140, 312–316 (1985). https://doi.org/10.1007/BF00446969
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00446969