Abstract
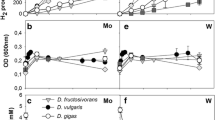
During growth of ethanol plus sulfate Desulfovibrio gigas and three other Desulfovibrio strains tested contained high NAD-dependent alcohol dehydrogenase activities and dye-linked aldehyde dehydrogenase activities. In lactate-grown cells these activities were lower or absent. In D. gigas an NADH dehydrogenase activity was found which was higher during growth on ethanol than during growth on lactate. The NADH dehydrogenase activity appeared to consist of at least three different soluble enzymes. The aldehyde dehydrogenase activity in D. gigas was highest with benzylviologen as an acceptor and was strongly stimulated by potassium ions. Coenzyme A or phosphate dependency could not be shown, indicating that acetyl-CoA or acetyl phosphate are not intermediates in the conversion of acetaldehyde to acetate.
In the absence of sulfate D. gigas was able to convert ethanol to acetate by means of interspecies hydrogen transfer to a methanogen. This conversion, however, did not lead to growth of the Desulfovibrio.
Similar content being viewed by others
Abbreviations
- DH:
-
dehydrogenase
- BV2+/BV+ :
-
oxidized/reduced benzylviologen
- DCPIP:
-
2,6-dichlorophenolindophenol
- MTT:
-
3-(4′,5′-dimethylthiazol-2-yl)-2,4-diphenyltetrazolium bromide
- MV2+/MV+ :
-
oxidized/reduced methylviologen
- PMS:
-
phenazine methosulfate
References
Adachi O, Matsushita, K, Shinagawa E, Ameyama M (1980a) Crystallization and properties of NADP-dependent aldehyde dehydrogenase from Gluconobacter melanogenus. Agric Biol Chem 44:155–164
Adachi O, Tayama K, Shinagawa E, Matsushita K, Ameyama M (1980b) Purification and characterization of membrane-bound aldehyde dehydrogenase from Gluconobacter suboxidans. Agric Biol Chem 44:503–515
Ameyama M, Osada K, Shinagawa E, Matsushita K, Adachi O (1981) Purification and characterization of aldehyde dehydrogenase of Acetobacter aceti. Agric Biol Chem 45:1889–1890
Attwood MM, Harder W (1974) The oxidation and assimilation of C2 compounds by Hyphomicrobium sp. J Gen Microbiol 84:350–356
Bellion E, Wu GTS (1978) Alcohol dehydrogenase from a facultative methylotrophic bacterium. J Bacteriol 135:251–258
Bryant MP, Wolin EA, Wolin MJ, Wolfe RS (1967) Methanobacillus omelianskii a symbiotic association of two species of bacteria. Arch Mikrobiol 59:20–31
Bryant MP, Campbell LL, Reddy CA, Crabill MR (1977) Growth of Desulfovibrio in lactate or ethanol media low in sulfate in association with H2-utilizing methanogenic bacteria. Appl Environ Microbiol 33:1162–1169
Duine JA, Frank Jzn J, Jongejan JA (1986) PQQ and quinoprotein enzymes in microbial oxidations. FEMS Microbiol Rev 32:165–178
Goa J (1953) A microbiuret method for protein determination; determination of total protein in the cerebrospinal fluid. Scand J Clin Lab Invest 5:218–222
Groeneveld A, Dijkstra M, Duine JA (1984) Cyclopropanol in the exploration of bacterial alcohol oxidation. FEMS Microbiol Lett 25:311–314
Howard BH, Hungate RE (1976) Desulfovibrio of the sheep rumen. Appl Environ Microbiol 32:598–602
Jakoby WB (1963) Aldehyde dehydrogenases. In: Boyer PD, Lardy H, Myrbäck K (eds) The enzymes. Academic Press, New York, pp 203–221
Jendrossek D, Steinbüchel A, Schlegel HG (1987) Three different proteins exhibiting NAD-depenent acetaldehyde dehydrogenase activity from Alcaligenes eutrophus. Eur J Biochem 167:541–548 (1987)
Kremer DR, Hansen TA (1987) Glycerol and dihydroxyacetone dissimilation in Desulfovibrio strains. Arch Microbiol 147:249–256
Laanbroek HJ, Abee T, Voogd IL (1982) Alcohol conversions by Desulfobulbus propionicus Lindhorst in the presence and absence of sulfate and hydrogen. Arch Microbiol 133:178–184
LeGall J (1968) Purification partielle et étude de la NAD: rubrédoxin oxydo-réductase de D. gigas. Ann Inst Pasteur 114:109–115
LeGall J, Fauque G (1988) Dissimilatory reduction of sulfur compounds. In: Zehnder AJB (ed) Biology of anaerobic microorganisms, chapter 11. John Wiley and Sons, New York London (in press)
Moura JJG, Xavier AV, Bruschi M, LeGall J, Hall DO, Cammack R (1976) A molybdenum-containing iron-sulfur protein from D. gigas. Biochem Biophys Res Commun 72:782–789
Nanninga HJ, Gottschal JC (1986) Isolation of a sulfate-reducing bacterium growing with methanol. FEMS Microbiol Ecol 38:125–130
Nanninga HJ, Gottschal JC (1987) Properties of Desulfovibrio carbinolicus sp. nov. and other sulfate-reducing bacteria isolated from an anaerobic-purification plant. Appl Environ Microbiol 53:802–809
Odom JM, Peck HD (1981) Localization of dehydrogenases, reductases and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol 147:161–169
Oser BL (1965) Determination of pyruvic acid. In: Oser BL (ed) Hawk's physiological chemistry. McGraw-Hill Book Company, New York Toronto Sydney London, pp 1107–1108
Peck HD, Lissolo T (1988) Assimilatory and dissimilatory sulphate reduction: enzymology and bioenergetics. In: Cole JA, Ferguson SJ (eds) The nitrogen and sulphur cycles. Cambridge University Press, Cambridge
Poels PA, Groen BW, Duine JA (1987) NAD(P)+-independent aldehyde dehydrogenase from Pseudomonas testosteroni A novel type of molybdenum-containing hydroxylase. Eur J Biochem 166:575–579
Postgate JR (1984) The sulphate-reducing bacteria. Cambridge University Press, Cambridge
Reddy CA, Bryant MP, Wolin MJ (1972) Ferredoxin-dependent conversion of acetaldehyde to acetate and H2 in extracts of S organism. J Bacteriol 110:133–138
Schink B (1985) Fermentation of acetylene by an obligate anaerobe, Pelobacter propionicus sp. nov. Arch Microbiol 142:295–301
Schink B, Kremer DR, Hansen TA (1987) Pathway of propionate formation from ethanol in Pelobacter propionicus. Arch Microbiol 147:321–327
Smith LT, Kaplan NO (1980) Purification, properties, and kinetic mechanism of coenzyme A-linked aldehyde dehydrogenase from Clostridium kluyveri. Arch Biochem Biophys 203:663–675
Stams AJM, Hansen TA (1986) Metabolism of l-alanine in Desulfotomaculum ruminis and two marine Desulfovibrio strains. Arch Microbiol 145:277–279
Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hansen TA (1984) Pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol 139:167–173
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Trüper HG, Schlegel HG (1964) Sulphur metabolism in Thiorhodaceae. I. Quantitative measurements of growing cells of Chromatium okenii. Antonie van Leeuwenhoek J Microbiol Serol 30:225–238
Turner N, Barata B, Bray RC, Deistung J, LeGall J, Moura JJG (1987) The molybdenum iron-sulphur protein from Desulfovibrio gigas as a form of aldehyde oxidase. Biochem J 243:755–761
Widdel F (1988) Microbiology and ecology of sulfate and sulfur-reducing bacteria. In: Zehnder AJB (ed) Biology of anaerobic microorganisms, chapter 10, John Wiley and Sons, New York London (in press)
Williamson DH (1974) L-alanine. Determination with alanine dehydrogenase. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Verlag Chemie, Weinheim, pp 1679–1682
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kremer, D.R., Nienhuis-Kuiper, H.E. & Hansen, T.A. Ethanol dissimilation in Desulfovibrio . Arch. Microbiol. 150, 552–557 (1988). https://doi.org/10.1007/BF00408248
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00408248