Summary
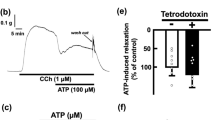
Nicorandil (10 μmol/l–0.3 mmol/l) and ATP (1 μmol/l–0.1 mmol/l) hyperpolarized the membrane of circular smooth muscle of the guinea-pig small intestine and increased conductance of the membrane probably to K ions as estimated by the effect on the current-voltage relationship. In the presence of a maximally hyperpolarizing concentration of nicorandil (0.1 mmol/l), ATP produced a further hyperpolarization of 5 mV. The ATP-induced but not the nicorandil-induced hyperpolarization required the presence of Ca in the medium, and the ATP-induced hyperpolarization was blocked by apamin treatment (1 nmol/l) or by MnCl2 (1.3 mmol/l). On the other hand, both hyperpolarization responses were blocked by the local anaesthetics procaine (0.1–1 mmol/l), lidocaine (0.1–1 mmol/l) or cocaine (0.3–1 mmol/l), with different potencies. Field stimulation of smooth muscle of the small intestine produced inhibitory junction potentials (i.j.p.s) and these were inhibited by apamin (10 nmol/l–100 nmol/l). In the presence of ATP, the amplitude of the i.j.p.s was markedly reduced, but in the presence of nicorandil the amplitude was only slightly reduced, consistent with the same increase in ionic conductance and hyperpolarization of the membrane. These results indicate that ATP and nicorandil hyperpolarize the membrane by activating different K-channels, i.e. Ca dependent and Ca insensitive K channels, respectively. As assessed from the effects of local anaesthetics and the membrane properties, the circular muscle may also possess other K channels different from the ATP and nicorandil sensitive K channels.
Similar content being viewed by others
References
Abe Y, Tomita T (1968) Cable properties of smooth muscle. J Physiol (Lond) 96:87–100
Baidan LV, Vladimirova IA, Mirochnikov AI, Tarah GA (1979) Deistivie apamin na sinapticheskviv peredachu v razlichnykh tipakh sinapsov. Dokl Akad Nauk USSR 241:1224–1227
Banks BEC, Brown C, Burgess GM, Burnstock G, Claret M, Cocks TM, Jenkinson DH (1979) Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature 282:415–417
Bauer V, Kuriyama H (1982) The nature of non-cholinergic, non-adrenergic transmission in longitudinal and circular muscles of the guinea-pig ileum. J Physiol (Lond) 332:375–391
Benham CD, Bolton TB, Lang RJ, Takewaki T (1985) The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+-channels in arterial and intestinal smooth muscle cell membranes. Pflügers Arch 403:120–127
Brading AF (1981) Ionic distribution and mechanism of transmembrane ion movements in smooth muscle. In: Bulbring E, Brading AF, Jones AW, Tomita T (eds) Smooth muscle. Arnold, London, pp 65–92
Burgess GM, Claret M, Jenkinson DH (1981) Effects of quinine and apamin on the calcium-dependent potassium permeability of mammalian hepatocytes and red cells. J Physiol (Lond) 317:67–90
Burnstock G (1980) Purinergic modulation of cholinergic transmission. Gen Pharmacol 11:15–18
Burnstock G, Campbell G, Satchell DG, Smythe A (1970) Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br J Pharmacol Chemother 40:668–688
Burnstock G, Dumsday B, Smythe A (1972) Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br J Pharmacol 44:451–461
Casteels R (1981) Membrane potential in smooth muscle. In: Bulbring E, Brading AF, Jones AW, Tomita T (eds) Smooth muscle. Arnold, London, pp 105–126
Dephour AR, Khoyi MA, Koutche KH, Zarrindast MR (1980) Pharmacological study of the anococcygeus muscle of the dog. Br J Pharmacol 71:35–40
Fujiwara S, Kuriyama H (1983) Effects of agents that modulate potassium permeability on smooth muscle cells of the guinea-pig basilar artery. Br J Pharmacol 79:23–35
Furukawa K, Itoh T, Kajiwara M, Kitamura K, Suzuki H, Ito Y, Kuriyama H (1981) Vasodilating actions of 2-nicotinamidoethyl nitrate on porcine and guinea-pig coronary arteries. J Pharmacol Exp Ther 218:248–259
Habermann E (1984) Apamin. Pharmacol Ther 25:255–270
Haezell MA (1975) Is ATP an inhibitory transmitter in the rat stomach? Br J Pharmacol 55:285–286
Hara Y, Kitamura K, Kuriyama H (1980) Actions of 4-amino-pyridine on vascular smooth muscle tissue of the guinea-pig. Br J Pharmacol 68:99–106
Hugues M, Romey G, Duval D, Vincent JP, Lazdunski M (1982) Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells; Voltage clamp and biochemical characterization of the tonic receptor. Proc Natl Acad Sci USA 79:1308–1312
Inoue R, Kitamura K, Kuriyama H (1985) Two Ca-dependent K channels classified by tetraethylammonium distribute on smooth muscle membranes of the rabbit portal vein. Pflügers Arch (in press)
Inoue T, Ito Y, Takeda K (1983) The effects of 2-nicotinamidoethyl nitrate on smooth muscle cells of the dog mesenteric artery and trachea. Br J Pharmacol 80:459–470
Inoue T, Kanmura Y, Fujisawa K, Itoh T, Kuriyama H (1984) Effects of 2-nicotinamidoethyl nitrate (Nicorandil; SG-75) and its derivatives on smooth muscle cells of the canine mesenteric artery. J Pharmacol Exp Ther 229:793–802
Ito Y, Kitamura K, Kuriyama H (1980) Effects of acetylcholine and catecholamine on the smooth muscle cells of the porcine coronary artery. J Physiol (Lond) 309:171–183
Itoh T, Furukawa K, Kajiwara M, Kitamura K, Suzuki H, Ito Y, Kuriyama H (1981) Effects of 2-nicotinamidoethyl nitrate on smooth muscle cells and on adrenergic transmission in the guinea-pig and porcine mesenteric arteries. J Pharmacol Exp Ther 218:260–270
Kajiwara M, Droogmans G, Casteels R (1984) Effects of 2-nicotinamidoethyl (Nicorandil) on excitation-contraction coupling in the smooth muscle cells of rabbit ear artery. J Pharmacol Ther 230:462–468
Karashima T, Takata Y (1979) The effects of ATP and related compounds on the electrical activity of the rat portal vein. Gen Pharmacol 10:477–487
Karashima T, Itoh T, Kuriyama H (1982) Effects of 2-nicotinamidoethyl nitrate on smooth muscle cells of the guinea-pig mesenteric and portal veins. J Pharmacol Exp Ther 221:472–480
Keatinge WR (1976) Effects of local anesthetics on electrical activity and voltage-dependent K-permeability of arteries. In: Worcel M, Vassort G (eds) Smooth muscle pharmacology and physiology. 50, pp 177–182
Kuriyama H, Ito Y, Suzuki H, Kitamura K, Itoh T (1982) Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol 243:H641-H662
Maas AJJ (1981) The effects of apamin on responses evoked by field stimulation on guinea-pig taenia-caeci. Eur J Pharmacol 73:1–9
Maas AJJ, DenHertog A (1979) The effects of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur J Pharmacol 58:151–156
MacKay D, McKirdy HC (1972) Effects of vasopressin and of adenosine triphosphate on the flat preparation of rabbit ileum. Br J Pharmacol 44:366–367
Ohga A, Taneike T (1977) Dissimilarity between the responses to adenine triphosphate or its compounds and non-adrenergic inhibitory nerve stimulation in the longitudinal muscle of the stomach. Br J Pharmacol 60:221–231
Takata Y, Kuriyama H (1980) ATP induced hyperpolarization of smooth muscle cells of the guinea-pig coronary artery. J Pharmacol Exp Ther 212:519–526
Tomita T, Watanabe H (1973) A comparison of the effect of adenosine triphosphate with noradranaline and with the inhibitory potential of the guinea-pig taenia-coli. J Physiol (Lond) 231:167–177
Vladimirova A, Shuba MF (1978) Strychnine, hydrastine and apamin effects on synaptic transmission in smooth muscle cells. Neurofiziologiya 10:296–299
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yamanaka, K., Furukawa, K. & Kitamura, K. The different mechanisms of action of nicorandil and adenosine triphosphate on potassium channels of circular smooth muscle of the guinea-pig small intestine. Naunyn-Schmiedeberg's Arch. Pharmacol. 331, 96–103 (1985). https://doi.org/10.1007/BF00498857
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00498857