Summary
The purpose of this study was to determine if the nonpeptide angiotensin II-1 receptor antagonist DuP 753 after, acute or chronic administration in vivo or after in vitro exposure, altered indices of dopaminergic function in rat striatum. In vivo studies examined the effect of acute and chronic 21-day administration of DuP 753 (10 mg/kg, s.c.) on levels of dopamine (DA) and its metabolite, dihydroxyphenylacetic acid (DOPAC). To determine if chronic treatment with DuP 753 was able to inhibit the pressor response to angiotensin II, a single i.v. dose of angiotensin II (0.1 μg/kg) was administered 18 hours after the last dose of DuP 753. Acute DuP 753 resulted in significantly decreased (14%) levels of DA. Chronic DuP 753 resulted in increased (1.64 fold) levels of DOPAC, although DA levels were not altered. The single i.v. administration of angiotensin II resulted in increased (88%) DOPAC levels regardless of chronic DuP 753.
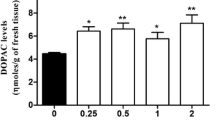
The in vitro effect of DuP 753 (0.1 nM–1.0 μM) on basal and field stimulation-evoked release of DA and DOPAC was determined in superfused striatal slices from drug naive rats. DA was not detected in these experiments. DuP 753 did not alter basal outflow of DOPAC. At low concentrations (1.0–10 nM), DuP 753 decreased (53%) stimulation-evoked DOPAC overflow; however, at concentrations greater than 10 nM, the inhibitory effect was diminished. Nomifensine (10 μM; a DA uptake inhibitor) was included in the superfusion buffer in order to measure the effect of DuP 753 on the concentration of DA in superfusate. DuP 753 had no effect on basal DA and DOPAC outflow. Nomifensine markedly potentiated the DuP 753-induced decrease in stimulation-evoked DOPAC and DA overflow. Pargyline (10 μM; a monoamine oxidase (MAO) inhibitor) was included with nomifensine in the superfusion buffer to examine the contribution of MAO to the DuP 753-induced decrease in dopaminergic neurotransmission. The effect of DuP 753 on DA overflow was not altered by the presence of pargyline. Additionally, angiotensin II (1 and 10 μM) increased the overflow of DOPAC from striatal slices under control conditions. Therefore, the results in vitro suggest that acutely the agonist increases DA neurotransmission and the antagonist decreases DA neurotransmission. In contrast, chronic in vivo adminitration of DuP 753 resulted in increased striatal DOPAC levels indicative of an increased dopaminergic neurotransmission. Therefore, chronic in vivo administration of DuP 753 appears to result in a compensatory response of the dopaminergic system in striatum.
Similar content being viewed by others
References
Anderson WP, Woods RL (1987) Intrarenal effects of angiotensin II in renal artery stenosis. Kidney Int 31 [Suppl 20] S151-S167
Bardelay C, Mach E, Worcel M, Hunt P (1989) Angiotensin-converting enzyme in rat brain and extraneural tissues visualized by quantitative autoradiography using 3H Trandolaprilate. J Cardiovasc Pharmacol 14:511–518
Bennett JP, Synder SH (1976) Angiotensin II binding to mammalian brain membranes. J Biol Chem 251:7423–7430
Brownfield MS, Reid IA, Ganten D, Ganong WF (1982) Differential distribution of immunoreactive angiotensin and angiotensin-converting enzyme in rat brain. Neuroscience 7:1759–1769
Chang RSL, Lotti VJ, Chen TB, Faust KA (1990) Two angiotensin II binding sites in rat brain revealed using [125I] SAR1, ILE8-angiotensin II and selective nonpeptide antagonists. Biochem Biophys Res Commun 171:813–817
Chiu AT, McCall DE, Price WA, Wong PC, Carini DJ, Duncia JV, Wexler RR, Yoo SE, Johnson AL, Timmermans PBMWM (1990) Nonpeptide angiotensin II receptor antagonists. VII. Cellular and biochemical pharmacology of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 252:711–718
Deschepper CF, Flaxman M (1990) Glucocorticoid regulation of rat diencephalon angiotensinogen production. Endocrinology 126:963–970
Dubocovich ML, Zahniser NR (1985) Binding of the dopamine uptake inhibitor 3H-nomifensine to striatal membranes. Biochem Pharmacol 34:1137–1144
Dwoskin LP, Zahniser NR (1986) Robust modulation of (3H) dopamine release from rat striatal slices by D-2 dopamine receptors. J Pharmacol Exp Ther 239:442–453
Ferrario C (1983) Neurogenic actions of angiotensin II. Hypertension 5 [Suppl V]:V73-V79
Fitzsimons JT, Setler PE (1975) The relative importance of the central nervous catecholaminergic and cholinergic mechanisms in drinking in response to angiotensin and other thirst stimuli. J Physiol 250:613–631
Fredholm BB, Dunwiddie TV, Bergman B, Lindstrom R (1984) Levels of adenosine and adenine nucleotides in slices of rat hippocampus. Brain Res 295:127–136
Gehlert DR, Speth RC, Wamsley JK (1986) Distribution of [125I] Angiotensin II binding sites in the rat brain: A quantitative autoradiographic study. Neuroscience 18:837–856
Gehlert DR, Gackenheimer SL, Reel JK, Lin HS, Steinberg MI (1990) Nonpeptide angiotensin II receptor antagonists discriminate subtypes of [125I]-angiotensin II binding sites in the rat brain. Eur J Pharmacol 187:123–126
Gerhardt GA, Dwoskin LP, Zahniser NR (1989) Outflow and overflow of picogram level of endogenous dopamine and DOPAC from rat striatal slices: Improved methodology for studies of stimulus-evoked release and metabolism. J Neurosci Methods 26:217–227
Hollenberg NK (1979) Pharmacological interruption of the reninangiotensin system. Ann Rev Pharmacol Toxicol 19:559–582
Lynch KR, Simnad VI, Ben-Ari ET, Garrison JC (1986) Localization of preangiotensinogen messenger RNA sequences in the rat brain. Hypertension 8:540–543
Mendelsohn FAO, Allen AM, Chai S-Y, McKinley MJ, Oldfield BJ, Paxinos G (1990) The brain angiotensin system: Insights from mapping its components. Trends Endocrinol MetabVol I, 4:189–198
Mendelsohn FAO, Quirion R, Saavedra JM, Aquilera G, Catt KJ (1984) Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci USA 81:1575–1579
Phillips MI (1978) Angiotensin in the Brain. Neuroendocrinology 25:354–377
Phillips MI, Stenstrom B (1985) Angiotensin II in rat brain comigrates with authentic angiotensin II in high pressure liquid chromatography. Circ Res 56:212–219
Quinlan JT, Phillips MI (1981) Immunoreactivity for an angiotensin II-like peptide in the human brain. Brain Res 205:212–218
Richoux JP, Bouhnik J, Clauser E, Corvol P (1988) The renin-angiotensin system in rat brain. Immunocytochemical localization of angiotensinogen in glial cells and neurons. Histochemistry 89:323–331
Simmonet G, Giorguieff-Chesselet MF, Caragon A, Bioulac B, Cesselin F, Glowinski J, Vincent JD (1979) Angiotensin II and the nigroneostriatal system. J Physiol (Paris) 77:71–79
Sirett NE, McLean AS, Bray JJ, Hubbard JI (1977) Distribution of angiotensin II receptors in rat brain. Brain Res 122:299–312
Soltis E, Jewell A, Dwoskin L, Cassis L (1992) DuP 753 treatment alters blood pressure and vascular reactivity in normotensive rats. Hypertension (in press)
Starke K (1977) Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol 77:1–124
Strittmatter SM, Lo MMS, Javitch JA, Snyder SH (1984) Autoradiographic localization of angiotensin-converting enzyme in rat brain with [3H]captopril: Location to striatonigral pathway. Proc Natl Acad Sci USA 81:1599–1603
Sumners C, Woodruff GN, Poat JA, Munday KA (1979) The effect of neuroleptic drugs on drinking induced by central administration of angiotensin or carbachol. Psychopharmacology 60:291–294
Volicer L, Loew CG (1971) Penetration of angiotensin II into the brain. Neuropharmacology 10:631–636
Wamsley JK, Herblin WF, Hunt M (1990) Use of non-peptide angiotensin II receptor antagonists to demonstrate angiotensin II receptor subtypes in brain. Soc Neurosci Abstr 16:685
Westfall TC (1977) Local regulation of adrenergic neurotransmission. Physiol Rev 57:659–728
Wong PC, Price WA, Chin AT, Duncia JV, Carini DJ, Wexler RR, Johnson AL, Timmermans PBMWM (1990a) Nonpeptide angiotensin II receptor antagonists. VIII. Characterization of functional antagonism displayed by DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 252:719–725
Wong PC, Hart SD, Zaspel AM, Chiu AT, Ardecky RJ, Smith RD, Timmermans PBMWM (1990b) Functional studies of nonpeptide angiotensin II receptor subtype specific ligands: DuP 753 (angiotensin II-1) and PD 123177 (angiotensin II-2). J Pharmacol Exp Ther 255:584–592
Wong PC, Price WA, Chiu AT, Duncia JV, Carini DJ, Wexler RR, Johnson AL, Timmermans PBMWM (1990c) Nonpeptide angiotensin II receptor antagonists. IX. Antihypertensive activity in rats of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 252:726–732
Wong PC, Price WA, Chiu AT, Duncia JV, Carini DJ, Wexler RR, Johnson AL, Timmermans PBMWM (1990d) Nonpeptide angiotensin II receptor antagonists. XI. Pharmacology of EXP 3174: An active metabolite of DuP 753, an orally active antihypertensive agent. J Pharmacol Exp Ther 255:211–217
Author information
Authors and Affiliations
Additional information
Send offprint requests to L.P. Dwoskin at the above address
This study was supported by a grant from the American Heart Association (Local Kentucky Affiliate). Portions of the results were presented in preliminary form at the Federation of American Society for Experimental Biology in Atlanta, GA, April, 1991
Rights and permissions
About this article
Cite this article
Dwoskin, L.P., Jewell, A.L. & Cassis, L.A. DuP 753, a nonpeptide angiotensin II-1 receptor antagonist, alters dopaminergic function in rat striatum. Naunyn-Schmiedeberg's Arch Pharmacol 345, 153–159 (1992). https://doi.org/10.1007/BF00165730
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00165730