Abstract
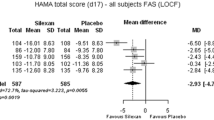
The selective 5HT3 antagonist tropisetron was studied in 91 outpatients meeting DSM-III criteria for Generalized Anxiety Disorder. Following a placebo washout period of up to 1 week, one of three active treatments (tropisetron 0.5 mg, 5 mg, or 25 mg daily) or placebo was given for a further 3 weeks. After 7 days treatment termination rates due to inefficacy showed a statistically significant dose-related therapeutic effect of tropisetron. Similar effects were seen on the Hopkins Symptom Check List total score and the Global Impression Scale. The Hamilton Anxiety Scale showed a similar trend which, however, failed to reach statistical significance. At day 21 tropisetron showed significant dose-dependent effects on all anxiety-related outcome measures. The incidence of adverse events was low and the severity generally mild. Most frequent complaints were headache, nausea, constipation and nervousness. Laboratory tests and physical examination performed at baseline and study end showed no significant treatment effects.
Similar content being viewed by others
References
American Psychiatric Association (1980) Diagnostic and statistical manual of mental disorders. American Psychiatric Association, Washington, DC
Bech P, Allerup P, Maier W, Albus M, Lavori P, Ayuso JL (1992) The Hamilton Scales and the Hopkins Symptom Checklist (SCL-90). A cross-national validity study in patients with panic disorders. Br J Psychiatry 160:206–211
Chen Y, Lader M (1990) Long-term benzodiazepine treatment: is it ever justified? Hum Psychopharm 5:301–312
Covi L, Lipman RS, McNair DM, Czerlinsky T (1979) Symptomatic volunteers in multicentre drug trials. Prog Neuropsychopharmacol 3:521–527
Cutler MG, Dixon AK (1988) Effects of the 5HT3 antagonist ICS 205-930 on behaviour of mice during social encounters. Br J Pharmacol 96:12
Derogatis LR (1977) The SCL-90 manual I: scoring, administration and procedures for the SCL-90. Clinical Psychometric Research, Baltimore
Evans C, Dolan B, Lynch D (1992) Reports of the death of factor analysis are greatly exaggerated (letter). Br J Psychiatry 161:272–273
Hollander M, Wolfe DA (1973) Nonparametric statistical methods. Wiley, New York
Lecrubier Y, Puech AJ, Azcona A (1990) 5HT3 receptors in anxiety disorders. Summer Meeting, abstract 19, British Association for Psychopharmacology, Cambridge
Maier W, Buller R, Philpp M, Heuser I (1988) The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. J Affective Disord 14:61–68
Mantel N (1963) Chi square tests with one degree of freedom. Extensions of the Mantel-Haenszel Procedure. JASA 58:690–700
Marsden CA (1990) The pharmacology of new anxiolytics acting on 5-HT neurones. Postgrad Med J 66 [Suppl 2]:S2-S6
Montgomery SA, Asberg M (1979) A new depression rating scale designed to be sensitive to change. Br J Psychiatry 134:382–389
National Institute of Mental Health (1976a) Hamilton Anxiety Scale. In: ECDEU assessment manual for psychopharmacology, Guy W, (ed), National Institute of Mental Health, Rockville, Md, pp 193–198
National Institute of Mental Health (1976b) Clinical Global Impression. In: Guy W, (ed) ECDEU assessment manual for psychopharmacology. National Institute of Mental Health, Rockville, Md, pp 217–222
Rasch G (1960) Probabilistic models for some intelligence and attainment tests. Danish Institute of Educational Research, Copenhagen
Raskin A, Schulterbrand J, Reatig N, McKeon JT (1969) Replication of factors of psychopathology in interview, ward behaviour and self report ratings of hospitalized depressives. J Nerv Ment Dis 148:87–98
Shaffer JP (1986) Modified sequentially rejective multiple test procedures. JASA 81:826–831
Author information
Authors and Affiliations
Additional information
(Drs Braconnier, Combes Lepastier, Danic, Deyrieux, Fabiani, Lazartigues, Peyrouzet, Raikovic and Supino-Viterbo)
Rights and permissions
About this article
Cite this article
Lecrubier, Y., Puech, A.J., Azcona, A. et al. A randomized double-blind placebo-controlled study of tropisetron in the treatment of outpatients with generalized anxiety disorder. Psychopharmacology 112, 129–133 (1993). https://doi.org/10.1007/BF02247373
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02247373