Abstract.
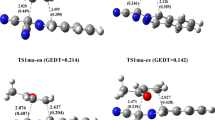
Acetylene insertion into Pt(II)–H and Pt(II)SiH3 bonds of PtH(SiH3)(PH3) was investigated using ab initio molecular orbital and Møller-Plesset perturbation theory methods. The insertion into PtH was predicted to proceed with a smaller activation energy (E a =12.8 kcal/mol) than that into PtSiH3 (E a =20.9 kcal/mol). The reaction energy (ΔE) of the insertion into PtH is 10 kcal/mol smaller than that for the insertion into PtSiH3, which reflects differences in bond energies between CH and CSi and between PtH and PtSiH3. A comparison with ethylene insertion revealed that the acetylene insertion occurs more easily, and the latter reaction is more exothermic. A simple vibronic coupling model combined with Toyozawa's interaction mode analysis was used to examine interesting differences in E a and ΔE between insertions into PtH and PtSiH3, and between acetylene and ethylene insertions. This analysis suggests that the factors determining E a are the stiffness of the PtH and PtSiH3 bonds and the vibronic coupling strength of acetylene and ethylene.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 13 August 1998 / Accepted: 2 September 1998 / Published online: 15 February 1999
Rights and permissions
About this article
Cite this article
Sugimoto, M., Yamasaki, I., Mizoe, N. et al. Acetylene-insertion reactions into Pt(II)–H and Pt(II)–SiH3 bonds. An ab initio MO study and analysis based on the vibronic coupling model. Theor Chem Acc 102, 377–384 (1999). https://doi.org/10.1007/s002140050509
Issue Date:
DOI: https://doi.org/10.1007/s002140050509