Abstract
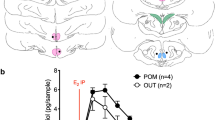
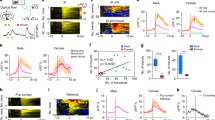
Electrical stimulation of the medial amygdala (AMY) elicited antidromic action potentials in neurons in the preoptic area (POA) and the lateral septum (LS) of 36 urethane-anesthetized ovariectomized female rats, which were either treated with estrogen o not treated. The extracellular potentials from the two sites showed similar characteristics, with the exception of the sensitivity to estrogen: they had latencies between 3 and 35 ms. Thresholds were as low as 100 μA. The mean relative refractory period was 2.2 ms. The peak-to-peak amplitudes of the positive-negative biphasic potential ranged from 1.0 mV to 12.0 mV. Estrogen had site-specific effects on parameters of antidromic activation in the POA. Estrogen-treated rats had a significantly higher threshold (937 vs 664 μA) and a longer refractory period (2.5 vs 2.1 ms) than the ovariectomized rats (P < 0.05 for each). The effects were absent in the LS. Selective cutting of the stria terminalis diminished the AMY-induced antidromic responses in the POA and LS. Electrical stimulation of the stria blocked the AMY-induced antidromic potentials by collision. Thus, estrogen-sensitive POA efferents as well as non-estrogen-sensitive LS efferents project to the AMY via the stria terminalis. Reductions in axonal excitability would inhibit neural conduction and transmission. Estrogen may therefore reduce the AMY inputs from the POA, without affecting those from the LS. Such alterations in the neural impulse flow may underlie estrogen-dependent neuroendocrine or behavioral regulation.
Similar content being viewed by others
References
Akaishi T, Sakuma Y (1985a) Estrogen excites oxytocinergic, but not vasopressinergic cells in the paraventricular nucleus of female rat hypothalamus. Brain Res 335:302–305
Akaishi T, Sakuma Y (1985b) Gonadal steroid actions on the paraventricular magnocellular neurosecretory cells of the male rat. Neurosci Lett 54:91–96
Akaishi T, Sakuma Y (1986) Projections of oestrogen-sensitive neurones from the ventromedial hypothalamic nucleus of the female rat. J Physiol (Lond) 372:207–220
Blaustein JD, Lehman MN, Turcotte JC, Greene G (1992) Estrogen receptors in dendrites and axon terminals in the guinea pig hypothalamus. Endocrinology 131:281–290
Clarke WP, Goldfarb J (1989) Estrogen enhances a 5-HT1A response in hippocampal slices from female rats. Eur J Pharmacol 160:195–197
Corodimas KP, Morrell JI (1990) Estradiol-concentrating forebrain and midbrain neurons project directly to the medulla. JComp Neurol 291:609–620
de Olmos J, Alheid GF, Beltrano CA (1985) Amygdala. In: Paxinos G (ed) The rat nervous system. Academic, Sydney pp 223–334
DonCarlos LL, Monroy E, Morrell JI (1991) Distribution of estrogen receptor-immunoreactive cells in the forebrain of the female guinea pig. J Comp Neurol 305:591–612
Donevan SD, Ferguson AF (1990) The characteristics of medial septal neurons antidromically identified as projecting to the median eminence and their response to gonadal steroids. J Neuroendocrinol 2:575–581
Dudley CA, Lee Y, Moss RL (1990) Electrophysiological identification of a pathway from the septal area to the medial amygdala: sensitivity to estrogen and luteinizing hormone-releasing hormone. Synapse 6:161–168
Dyer RG (1973) An electrophysiological dissection of the hypothalamic regions which regulate the pre-ovulatory surge of luteinizing hormone in the rat. J Physiol (Lond) 234:421–442
Erulkar SD, Wetzel DM (1989) 5α-Dihydrotestosterone has nonspecific effects on membrane channels and possible genomic effects on ACh-activated channels. J Neurophysiol 61:1036–1052
Fahrbach SE, Morrell JI, Pfaff DW (1986) Identification of medial preoptic neurons that concentrate estradiol and project to the midbrain in the rat. J Comp Neurol 247:364–382
Hasegawa T, Sakuma Y (1993) Development effect of testosterone on estrogen sensitivity of the rat preoptic neurons with axons to the ventral tegmental area. Brain Res 611:1–6
Hoshina Y, Takeo T, Nakano K, Sato T, Sakuma Y (1994) Axonsparing lesion of the preoptic area enhances receptivity and diminishes proceptivity among components of female rat sexual behavior. Behav Brain Res 60:1–8
Karkanias GB, Etgen A (1993) Estradiol attenuates α2-adrenore-ceptor-mediated inhibition of hypothalamic norepinephrine release. J Neurosci 13:3488–3455
Kelly MJ, Moss RL, Dudley CA (1977) The effects of microiontophoretically applied estrogen, cortisol, and acetylcholine on medial preoptic-septal unit activity throughout the estrous cycle of the female rat. Exp Brain Res 30:53–64
Kendrick KM, Drewett RF (1980) Testosterone-sensitive neurones respond to oestradiol but not to dihydrotestosterone. Nature 286:67–68
Kevetter GA, Winans SS (1981) Connections of the corticomedial amygdala in the golden hamster. II Efferents of the ‘olfactory amygdala’. J Comp Neurol 197:99–111
King TR, Nance DM (1985) The effects of unilateral frontolateral hypothalamic knife cuts and asymmetrical unilateral septal lesions on lordosis behavior of rats. Physiol Behav 35:955–959
Masco DH, Carrer HF (1980) Sexual receptivity in female rats after lesion or stimulation in different amygdaloid nuclei. Physiol Behav 24:1073–1080
McEwen BS, Coirini H, Schumacher M (1990) Steroid effects on neuronal activity: when is the genome involved? In: Chadwick D, Widdows K (eds) Steroids and neuronal activity. Wiley, Chichester, pp 3–21
McGinnis M, Nance DM, Gorski RA (1978) Olfactory septal and amygdala lesions alone or in combination: effects on lordosis behavior and emotionality. Physiol Behav 20:435–440
McGregor A, Herbert J (1992) Differential effects of excitotoxic basolateral and corticomedial lesions of the amygdala on the behavioural and endocrine responses to either sexual or aggression-promoting stimuli in the male rat. Brain Res 574:9–20
Minami T, Oomura Y, Nabekura J, Fukuda A (1990) 17 β -Estradiol depolarization of hypothalamic neurons is mediated by cyclic AMP. Brain Res 519:301–307
Mobbs CV, Harlan RE, Burrous MR, Pfaff DW (1988) An estradiol-induced protein synthesized in the ventral medial hypothalamus and transported to the midbrain central gray. J Neurosci 8:113–118
Mobbs CV, Kaplitt M, Kow LM, Pfaff DW (1991) PLC-α: a common mediator of the action of estrogen and other hormones? Mol Cell Endocrinol 81:187–191
Morrell JI, Pfaff DW (1982) Characterization of estrogen-concentrating hypothalamic neurons by their axonal projections. Science 217:1273–1276
Morrell JI, Pfaff DW (1984) Hypothalamic and limbic system estradiol (E2) concentrating neurons that project to the amygdala. Soc Neurosci Abstr 10:210
Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A (1986) Mechanism of the rapid effect of 17 β -estradiol on medial amygdala neurons. Science 233:226–228
Olazabal UE, Pfaff DW, Mobbs CV (1992) Sex differences in the regulation of heat shock protein 70 kDa and 90 kDa in the rat ventromedial hypothalamus by estrogen. Brain Res 596:311–314
Ottersen OP (1980) Afferent connections to the amygdaloid complex of the rat and cat. II. Afferents from the hypothalamus and the basal telencephalon. J Comp Neurol 194:267–289
Pragnell M, Snay KJ, Trimmer JS, Maclusky NJ, Naftolin F, Kaczmarek LK, Boyle MB (1990) Estrogen induction of a small, putative K+ channel messenger RNA in rat uterus. Neuron 4:807–812
Rhodes CH, Morrell JI, Pfaff DW (1982) Estrogen-concentrating neurophysin-containing hypothalamic magnocellular neurons in the vasopressin-deficient (Brattleboro) rat: a study combining steroid autoradiography and immunocytochemistry. J Neurosci 2:1718–1724
Rodriguez del Castillo A, Battaner E, Guerra M, Alonso T, Mas M (1987) Regional changes of brain Na+, K+-transporting adenosine triphosphatase related to ovarian function. Brain Res 416:113–118
Russchen FT (1982) Amygdalopetal projections in the cat. II. Subcortical afferent connections. A study with retrograde tracing techniques. J Comp Neurol 207:157–176
Sakamoto Y, Suga S, Sakuma Y (1993) Estrogen-sensitive neurons in the female rat ventral tegmental area: a dual route for the hormone action. J Neurophysiol 70:1469–1475
Sakuma Y (1984) Influences of neonatal gonadectomy or androgen exposure on the sexual differentiation of the rat ventromedial hypothalamus. J Physiol (Lond) 349:273–286
Sakuma Y, Akaishi T (1987) Cell size, projection path, and localization of estrogen-sensitive neurons in the rat ventromedial hypothalamus. J Neurophysiol 57:1148–1159
Schiess MC, Joels M, Shinnick-Gallagher P (1988) Estrogen priming affects active membrane properties of medial amygdala neurons. Brain Res 440:380–385
Simerly RB, Swanson LW (1988) Projections of the medial preoptic nucleus: a phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol 270:209–242
Simerly RB, Chang C, Muramatsu M, Swanson LW (1990) Distribution of androgen and estrogen receptor messenger RNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol 294:76–95
Smith SS (1989) Estrogen administration increases neuronal responses to excitatory amino acids as a longterm effect. Brain Res 503:354–357
Suga S, Sakuma Y (1994) Dihydrotestosterone-sensitive neurons in the male rat ventromedial hypothalamus. Brain Res Bull 33:205–210
Swadlow HA, Waxman SG, Rosene DC (1978) Latency variability and the identification of antidromically activated neurons in mammalian brain. Exp Brain Res 32:439–443
Swanson LW, Cowan WM (1979) The connection of the septal region in the rat. J Comp Neurol 186:621–656
Szego CM, Pietras RJ (1981) Membrane recognition and effecter sites in steroid hormone action. Biochem Action Horm 8:307–463
Takeo T, Chiba Y, Sakuma Y (1993) Suppression of the lordosis reflex of female rats by efferents of the medial preoptic area. Physiol Behav 53:831–838
Towle A, Sze PY (1983) Steroid binding to synaptic plasma membrane: differential binding of glucocorticoids and gonadal steroids. J Steroid Biochem 18:135–143
Wong M, Moss RL (1992) Modulation of single-unit activity in the rat medial amygdala by neurotransmitters, estrogen priming, and synaptic inputs from the hypothalamus and midbrain. Synapse 10:94–102
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoshida, M., Suga, S. & Sakuma, Y. Estrogen reduces the excitability of the female rat medial amygdala afferents from the medial preoptic area but not those from the lateral septum. Exp Brain Res 101, 1–7 (1994). https://doi.org/10.1007/BF00243211
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00243211