Summary
-
1.
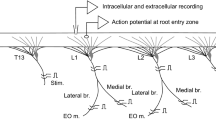
Responses of neck motoneurons to stimulation of the interstitial nucleus of Cajal (INC) were recorded intracellularly in cats under chloralose anesthesia. When stimuli were applied within or close to the INC, short latency, monosynaptic excitatory postsynaptic potentials (EPSPs) were evoked in many neck motoneurons. Such EPSPs were not evoked by stimulating mesencephalic regions outside the INC.
-
2.
Stimulation of the ipsilateral INC produced monosynaptic EPSPs consistently in biventer cervicis-complexus (BCC) motoneurons, while such EPSPs were observed in about two thirds of the splenius (SP) motoneurons and half of the trapezius (TR) motoneurons tested. Stimulation of the contralateral INC produced weak monosynaptic EPSPs in about half the BCC motoneurons and in a few SP and TR motoneurons. All types of motoneurons also received longer latency, apparently polysynaptic, PSPs from both INCs. In BCC and TR motoneurons these were mainly EPSPs, in SP, mixed excitatory and inhibitory PSPs.
-
3.
Monosynaptic EPSPs evoked by INC stimulation were not eliminated by acute and chronic parasagittal and transverse lesions placed to interrupt the bifurcating axons of all vestibulospinal and many reticulospinal neurons. No significant collision was observed between EPSPs evoked by INC and vestibular or reticular stimulation. The EPSPs evoked by stimulation of the INC therefore appear to have been produced by activation of interstitiospinal neurons rather than by an axon reflex mechanism.
-
4.
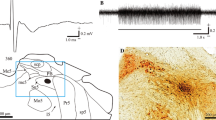
The properties of a number of interstitiospinal neurons were observed while recording extracellularly from the mesencephalon to map the location of the INC. One third of the interstitiospinal neurons activated antidromically from the C4 segment could also be activated antidromically from L1. These lumbar-projecting neurons had conduction velocities ranging from 15–123 m/s. Several interstitiospinal neurons sending axons to the ventral horn of the neck segments were identified and two of these were found to be branching neurons that projected both to the neck and to lower levels of the spinal cord.
Similar content being viewed by others
References
Abzug, C., Maeda, M., Peterson, B.W., Wilson, V.J.: Cervical branching of lumbar vestibulospinal axons. J Physiol (Lond) 243, 499–522 (1974)
Abzug, C., Peterson, B.W.: Antidromic stimulation in the pontomedullary reticular formation of local axon branches of contralateral vestibular neurons. Brain Res 64, 407–413 (1973)
Anderson, M.E., Yoshida, M., Wilson, V.J.: Influence of superior colliculus on cat neck motoneurons. J Neurophysiol 34, 898–907 (1971)
Berman, A.L.: The brain stem of the cat. A cytoarchitectonic atlas with stereotaxic coordinates, pp. 58–59. Madison, Milwaukee, London: The University of Wisconsin Press 1968
Bizzi, E., Kalil, R.E., Tagliasco, V.: Eye-head coordination in monkeys: Evidence for centrally patterned organization. Science 173, 452–454 (1971)
Brodai, A.: The reticular formation of the brain stem. Anatomical aspects and functional correlations, pp. 8–12. Edinburgh: Oliver and Boyd 1957
Büttner-Ennever, J.A., Henn, V.: An autoradiographic study of the pathway from the pontine reticular formation involved in horizontal eye movements. Brain Res 108, 155–164 (1976)
Cajal, S.R.Y. (1896): Indirect citation from A. Brodal's “Anatomy of the vestibular nuclei and their connections”. In: “Handbook of sensory physiology”. Vol. VI/1, pp. 210–352. Berlin, Heidelberg, New York: Springer 1974
Carpenter, M.B., Harbison, J.W., Peter, P.: Accessory oculomotor nuclei in the monkey: Projections and effects of discrete lesions. J Comp Neurol 140, 131–154 (1970)
Fukushima, K., Pitts, N.G., Peterson, B.W.: Responses of neck motoneurons to stimulation of the interstitial nucleus of Cajal. (Abstract) Ann Meeting Soc Neurosci 7, 154 (1977)
Graybiel, A.M.: Direct and indirect preoculomotor pathway of the brainstem: an autoradiographic study of the pontine reticular formation in the cat. J Comp Neurol 175, 37–78 (1977)
Hassler, R., Hess, W.R.: Experimentelle und anatomische Befunde über die Drehbewegungen und ihre nervösen Apparate. Arch Psychiatr Nervenkr 192, 488–526 (1954)
Hyde, J.E., Toczek, S.: Functional relation of interstitial nucleus to rotatory movements evoked from zona incerta stimulation. J Neurophysiol 25, 455–466 (1962)
Klüver, H., Barrera, E.: A method for the combined staining of cells and fibers in the nervous system. J Neuropathol Exp Neurol 12, 400–403 (1953)
Kuypers, H.G.J.M., Maisky, V.A.: Retrograde axonal transport of horseradish peroxidase from spinal cord to brain stem cell groups in the cats. Neurosci Letters 1, 9–14 (1975)
Ladpli, R., Brodal, A.: Experimental studies of the commissural and reticular formation projections from the vestibular nuclei in the cat. Brain Res 8, 65–96 (1968)
Markham, C.H., Precht, W., Shimazu, H.: Effect of stimulation of interstitial nucleus of Cajal on vestibular unit activity in the cat. J Neurophysiol 29, 493–507 (1966)
McMasters, R.E., Weiss, A.H., Carpenter, M.B.: Vestibular projections to the nuclei of the extraocular muscles. Degeneration resulting from discrete partial lesions of the vestibular nuclei in the monkey. Am J Anat 118, 163–194 (1966)
Nauta, W.J.H., Kuypers, H.G.J.M.: Some ascending pathways in the brain stem reticular formation. In: “Reticular formation of brain” Henry Ford Hospital Symposium. H.H. Jasper et al. (Eds.), pp. 3–30. Boston: Little and Brown 1958
Nyberg-Hansen, R.: Sites of termination of interstitiospinal fibers in the cat. An experimental study with silver impregnation methods. Arch Ital Biol 104, 98–111 (1966a)
Nyberg-Hansen, R.: Functional organization of descending supraspinal fibre systems to the spinal cord. Anatomical observations and physiological correlations. Ergebn. Anat Entwickl-Gesch 39/2, 1–48 (1966b)
Peterson, B.W., Anderson, M.E., Filion, M.: Responses of pontomedullary reticular neurons to cortical, tectal and cutaneous stimuli. Exp Brain Res 21, 19–44 (1974)
Peterson, B.W., Maunz, R.A., Pitts, N.G., Mackel, R.G.: Patterns of projection and branching of reticulospinal neurons. Exp Brain Res 23, 333–351 (1975)
Pompeiano, O., Walberg, F.: Descending connections to the vestibular nuclei. An experimental study in the cat. J Comp Neurol 108, 465–503 (1957)
Rall, W., Smith, T.G., Frank, K., Burke, R.E., Nelson, P.G.: Dendritic location of synapses and possible mechanisms for the monosynaptic EPSP in motoneurons. J Neurophysiol 30, 1169–1193 (1967)
Scheibel, M.E., Scheibel, A.B.: Structural substrates for integrative patterns in the brain stem reticular core. In: “Reticular formation of the brain” Henry Ford Hospital Symposium. H.H. Jasper et al. (Eds.), pp. 31–55. Boston: Little and Brown 1958
Schwindt, P.C., Precht, W., Richter, A.: Monosynaptic excitatory and inhibitory pathway from medial midbrain nuclei to trochlear motoneurons. Exp Brain Res 20, 223–238 (1974)
Shinoda, Y., Arnold, A.P., Asanuma, H.: Spinal branching of corticospinal axons in the cat. Exp Brain Res 26, 215–234 (1976)
Shinoda, Y., Ghez, C., Arnold, A.: Spinal branching of rubrospinal axons in the cat. Exp Brain Res 30, 203–218 (1977)
Szentàgothai, J.: Die zentrale Innervation der Augenbewegungen. Arch Psyhiatr Nervenkr 116, 721–760 (1943)
Toczek, S., Hyde, J.E.: Effect of vestibular nerve section on torsion and on evoked rotatory movements. Exp Neurol 8, 143–154 (1963)
Tarlov, E.: Organization of vestibulo-ocular projections in the cat. Brain Res 20, 159–179 (1970)
Thomas, R.C., Wilson, V.J.: Precise localization of Renshaw cells with a new marking technique. Nature 206, 211–213 (1965)
Wilson, V.J., Yoshida, M.: Monosynaptic inhibition of neck motoneurons by the medial vestibular nucleus. Exp Brain Res 9, 365–380 (1969)
Wilson, V.J., Wylie, R.M., Marco, L.A.: Organization of the medial vestibular nucleus. J Neurophysiol 31, 166–175 (1968)
Author information
Authors and Affiliations
Additional information
Supported in part by grants NSF BMS 75-00487 and NIH NS 02619
Recipient of N. I. H. Fellowship l F32 NS 05027
Rights and permissions
About this article
Cite this article
Fukushima, K., Pitts, N.G. & Peterson, B.W. Direct excitation of neck motoneurons by interstitiospinal fibers. Exp. Brain Res. 33, 565–581 (1978). https://doi.org/10.1007/BF00235575
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00235575