Summary
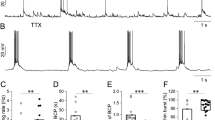
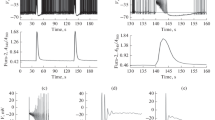
Lowering extracellular [Ca2+] in rat hippocampal slices induces spontaneous epileptiform activity in area CA1, which is characterized by rhythmic burst firing of CA1 neurons and by prolonged negative potential shifts at the pyramidal cell body layer. This activity is accompanied by transient decreases of [Na+] and increases of [K+] in the extracellular space. In spite of the complete blockade of synaptic transmission, the wave of epileptiform activity propagates across area CA1. These findings suggest, that non-synaptic mechanisms may play a role in the generation and spread of epileptiform activity in the mammalian CNS.
Similar content being viewed by others
References
Dietzel I, Heinemann U, Hofmeier G, Lux HD (1978) Stimulusinduced changes in extracellular Na+ and Cl− concentration in relation to changes in the size of the extracellular space. Exp Brain Res 46: 73–84
Erulkar SD, Rahamimoff R, Rotshenker R (1978) Quelling of spontaneous transmitter release by nerve impulses in low extracellular calcium solutions. J Physiol (Lond) 278: 491–500
Frankenhaeuser B, Hodgkin AL (1957) The action of calcium on the electrical properties of squid axons. J Physiol (Lond) 137: 218–244
Gjerstad L, Andersen P, Langmoen IA, Lundervold A, Hablitz J (1981) Synaptic triggering of epileptiform discharges in CA1 pyramidal cells in vitro. Acta Physiol Scand 113: 245–252
Heinemann U, Gutnick MJ (1979) Relation between extracellular potassium concentration and neuronal activities in cat thalamus (VPL) during projection of cortical epileptiform discharge. Electroenc Clin Neurophysiol 47: 345–357
Heinemann U, Konnerth A, Louvel J, Lux HD, Pumain R (1982) Changes in extracellular free Ca in normal and epileptic sensorimotor cortex of cats. In: Klee MR, Lux HD, Speckmann EJ (eds) Physiology and pharmacology of epileptogenic phenomena. Raven Press, New York, pp 29–35
Heinemann U, Lux HD (1983) Ionic changes during experimentally induced epilepsies. In: Rose FC (ed) Progress in epilepsy. Pitman Medical, London (in press)
Heinemann U, Lux HD, Gutnick MJ (1977) Extracellular free calcium and potassium during paroxysmal activity in cerebral cortex of the cat. Exp Brain Res 27: 237–243
Hofmeier G, Lux HD (1981) The time courses of intracellular free calcium and related effects after injection of CaCl2 into neurones of the snail, Helix pomatia. Pfluegers Arch 391: 242–251
Jefferys JGR, Haas HL (1982) Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature 300: 448–450
Katz B (1969) The release of neural transmitter substances. The Sherrington lecture. Thomas, Springfield, Ill.
Llinás R, Nicholson C (1974) Analysis of field potentials in the central nervous system. In: Remond A (ed) Handbook of EEG clinical neurophysiology, vol 2, part B. Elsevier, Amsterdam, pp 61–92
Lorente De No R (1934) Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol 46: 113–177
Malenka RC, Kocsis JD, Jeffery D, Ransom BR, Waxman SG (1981) Modulation of parallel fiber excitability by postsynaptic mediated changes in extracellular potassium. Science 214: 339–341
Millichap JG (1969) Systemic electrolyte and neuroendocrine mechanisms. In: Jasper HH, Ward AA, Pope A (eds) Basic mechanisms of the epilepsies. Little, Brown & Co., Boston, pp 709–729
Orkand RK, Nicholls JG, Kuffler SW (1966) Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J Neurophysiol 29: 788–806
Prince DA (1982) Epileptogenesis in hippocampal and neocortical neurons. In: Klee M, Lux HD, Speckmann EJ (eds) Physiology and pharmacology of epileptogenic phenomena. Raven Press, New York, pp 151–162
Schwartzkroin PA (1975) Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res 85: 423–436
Schwartzkroin PA, Prince DA (1978) Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res 147: 117–130
Skrede KK, Westgaard RH (1971) The transverse hippocampal slice: A well defined structure maintained in vitro. Brain Res 35: 589–593
Taylor CP, Dudek FF (1982) Synchronous neural afterdischarges in rat hippocampal slices without active chemical synapses. Science 218: 810–812
Author information
Authors and Affiliations
Additional information
Supported by DFG grants He 1128/2-2 and He 1128/3. Y.Y. was supported by an ETP training grant and by the Meller Endowment Fund
Rights and permissions
About this article
Cite this article
Yaari, Y., Konnerth, A. & Heinemann, U. Spontaneous epileptiform activity of ca1 hippocampal neurons in low extracellular calcium solutions. Exp Brain Res 51, 153–156 (1983). https://doi.org/10.1007/BF00236813
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00236813