Summary
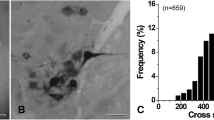
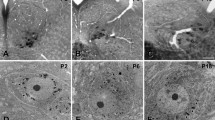
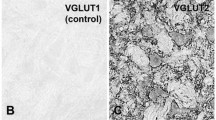
The purpose of this study was to define the types and distribution of synaptic terminals in the hypoglossal nucleus (XII) of the rat. Based on differences in bouton and vesicle size and shape, synaptic specializations and association with postsynaptic organelles, five types of terminals were identified in XII. In order of decreasing frequency they were: 1) S-boutons (spherical vesicles with an asymmetrical synapse); 2) F-boutons (flattened vesicles with a symmetrical synapse); 3) P-boutons (pleomorphic admixture of flattened and spherical vesicles with a symmetrical synapse); 4) C-boutons (pleomorphic vesicles with a subsynaptic cistern); and 5) Tboutons (spherical vesicles with an asymmetrical synapse and subsynaptic dense bodies). S-boutons were the predominant type found on dendrites, while boutons containing flattened vesicles were more prevalent on motoneuron somata. C-boutons were restricted exclusively to cell bodies and large dendrites, and T-boutons were seen primarily on smaller dendritic profiles. These results are, in general, comparable to those previously described in the ventral horn and cranial nerve motor nuclei in several species. However, differences were noted. Specifically, large M-boutons and axo-axonic synapses were not observed in the present study. The functional significance of these findings are discussed in relation to oro-lingual behaviour.
Similar content being viewed by others
References
Aldes LD, Boone TB (1984) A combined flat-embedding, HRP histochemical method for correlative light and electron microscopy study of single neurons. J Neurosci Res 11: 27–34
Alley KE (1973) Neuronal morphology and synaptic patterns in the hypoglossal nucleus. J Dent Res 52: 100
Altmann H, tenBruggencate G, Sonnhof U (1972) Differential strength of action of glycine and GABA in hypoglossal nucleus. Pflugers Arch 331: 90–94
Bak IJ, Choi WB (1974) Electron microscopic investigation of synaptic organization of the trochlear nucleus in the cat. I. Normal ultrastructure. Cell Tiss Res 150: 409–423
Barnard JW (1940) The hypoglossal complex of vertebrates. J Comp Neurol 72: 489–524
Bernstein JJ, Bernstein ME (1976) Ventral horn synaptology in the rat. J Neurocytol 5: 109–123
Bloom FE, Aghajanian GK (1968) An electron microscopic analysis of large granular vesicles of the brain in relation to monoamine content. J Pharmacol Exp Ther 159: 261–273
Bodian D (1966a) Synaptic types on spinal motoneurons: An electron microscopic study. Johns Hopkins Med J 119: 16–45
Bodian D (1966b) Electron microscopy: Two major synaptic types on spinal motoneurons. Science 151: 1093–1094
Bodian D (1970) An electron microscopic characterization of classes of synaptic vesicles by means of controlled aldehyde fixation. J Cell Biol 44: 115–124
Boone TB, Linner JG, Aldes LD (1982) Ultrastructural organization of the hypoglossal nucleus of the rat. Soc Neurosci Abstr 8: 875
Boone TB, Aldes LD (1984) The ultrastructure of two distinct neuron populations in the rat. Exp Brain Res 54: 321–326
Borke RC (1982) Perisomatic changes in the maturing hypoglossal nucleus after axon injury. J Neurocytol 11: 463–485
Chan-Palay V (1973) On certain fluorescent axon terminals containing granular synaptic vesicles in the cerebellar nucleus lateralis. Z Anat Entwickl-Gesch 142: 239–258
Conradi S (1969) On motoneuron synaptology in adult cats. Acta Physiol Scand Suppl 332: 5–48
Cooper MN (1981a) Neurons of the hypoglossal nucleus of the rat. Otolaryngol Head Neck Surg 89: 10–15
Cooper MN (1981b) The hypoglossal nucleus of the primate: A Golgi study. Neurosci Lett 21: 249–254
Destombes J, Gogan P, Rouvière A (1979) The fine structure of neurones and cellular relationships in the abducens nucleus in the cat. Exp Brain Res 35: 249–267
Dubner R, Sessle BJ, Storey AT (1978) The neural basis of oral and facial function. Plenum, New York, pp 290–303
Duggan AW, Lodge D, Biscoe TJ (1973) The inhibition of hypoglossal motoneurons by impulses in the glossopharyngeal nerve of the rat. Exp Brain Res 17: 261–270
Eccles JC (1964) The physiology of synapses. Academic Press, New York
Falls WM, King JS (1976a) The facial motor nucleus of the opossum: Cytology and axosomatic synapses. J Comp Neurol 167: 177–204
Falls WM, King JS (1976b) The facial motor nucleus of the opossum: Synaptic endings on dendrites. J Comp Neurol 167: 205–226
Gottlieb DI, Cowan WM (1972) On the distribution of axonal terminals containing spheroidal and flattened synaptic vesicles in the hippocampus and dentate gyrus of the rat and cat. Z Zellforsch 129: 413–429
Gregg RA, Carpenter DO (1982) Responses of hypoglossal motoneurons to various transmitters in a brain slice preparation. Soc Neurosci Abstr 8: 958
Hamos JE, King JS (1980) The synaptic organization of the motor nucleus of the trigeminal nerve in the opposum. J Comp Neurol 194: 441–463
Hashimoto PH, Palay SL (1965) Electron microscopy of the dorsal nucleus of the vagus, the hypoglossal nucleus, and the intermediolateral horn of the thoracic cord in rats and cats. Anat Rec 151: 360
Jones BE, Friedman L (1983) Atlas of catecholamine perikarya, varicosities and pathways in the brainstem of the cat. J Comp Neurol 215: 382–396
Kirkpatrick JB (1968) Chromatolysis in the hypoglossal nucleus of the rat: An electron microscopic analysis. J Comp Neurol 132: 189–212
Levitt P, Moore RY (1979) Origin and organization of brainstem catecholamine innervation in the rat. J Comp Neurol 186: 505–528
Matus AI, Dennison ME (1971) Autoradiographic localization of tritiated glycine at ‘flat-vesicle’ synapses in the spinal cord. Brain Res 32: 195–197
McLaughlin BJ (1972) The fine structure of neurons and synapses in the motor nuclei of the cat spinal cord. J Comp Neurol 144: 429–460
McLaughlin BJ, Wood JG, Saito K, Barber R, Vaughn JE, Roberts E, Wu JY (1974) The fine structural localization of glutamate decarboxylase in synaptic terminals of rodent cerebellum. Brain Res 76: 377–391
Nakajima Y, Reese TS (1983) Inhibitory and excitatory synapses in crayfish stretch receptor organs studied with direct rapidfreezing and freeze-substitution. J Comp Neurol 213: 66–73
Odutola AB (1976) Cell grouping and golgi architecture of the hypoglossal nucleus of the rat. Exp Neurol 52: 356–371
Okada Y, Nitsch-Hassler C, Kim JS, Bak IJ, Hassler R (1971) Role of gamma-aminobutyric acid (GABA) in the extrapyramidal motor system. I. Regional distribution of GABA in rabbit, rat, guinea pig and baboon CNS. Exp Brain Res 13: 514–518
Peters A, Palay SL, Webster HdeF (1976) The fine structure of the nervous system. W B Saunders Co, Philadelphia
Schmidt RF (1971) Presynaptic inhibition in the vertebrate central nervous system. Ergebn Physiol 63: 20–101
Smith TG, Wuerker RB, Frank K (1967) Membrane impedance changes during synaptic transmission in cat spinal motoneurons. J Neurophysiol 30: 1072–1096
Spencer RF, Sterling P (1977) An electron microscope study of motoneurons and interneurons in the cat abducens nucleus identified by retrograde intraaxonal transport of horseradish peroxidase. J Comp Neurol 176: 65–86
Sumner BEH (1975a) A quantitative study of subsurface cisterns and their relationships in normal and axotomized hypoglossal neurons. Exp Brain Res 22: 175–183
Sumner BEH (1975b) A quantitative analysis of the response of presynaptic boutons to postsynaptic motoneuron axotomy. Exp Neurol 46: 605–615
Sumner BEH (1975c) A quantitative analysis of boutons with different types of synapse in normal and injured hypoglossal nuclei. Exp Neurol 49: 406–417
Sumner BEH, Sutherland FI (1973) Quantitative electron microscopy on the injured hypoglossal nucleus in the rat. J Neurocytol 2: 315–328
tenBruggencate G, Sonnhof U (1972) Glycine and GABA, and blocking effects of strychnine and picrotoxin in the hypoglossal nucleus. Pflügers Arch 334: 240–252
Tredici G, Pizzini G, Milanesi S (1976) The ultrastructure of the oculomotor nerve (somatic efferent portion) of the cat. Anat Embryol 149: 323–346
Uchizuno K (1965) Characteristics of excitatory and inhibitory synapses in the central nervous system of the cat. Nature 207: 642–643
Valdivia O (1971) Methods of fixation and the morphology of synaptic vesicles. J Comp Neurol 142: 257–274
Valverde F (1961) Reticular formation of the pons and medulla: A golgi study. J Comp Neurol 116: 71–99
Young AB, Oster-Granite ML, Herndon RM, Snyder SH (1974) Glutamic acid: Selective depletion by viral induced granule cell loss in hamster cerebellum. Brain Res 73: 1–13
Zarbin MA, Wamsley JK, Kuhar MJ (1981) Glycine receptor: Light microscopic autoradiographic localization with [3H]-strychnine. J Neurosci 1: 532–547
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boone, T.B., Aldes, L.D. Synaptology of the hypoglossal nucleus in the rat. Exp Brain Res 57, 22–32 (1984). https://doi.org/10.1007/BF00231128
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00231128