Summary
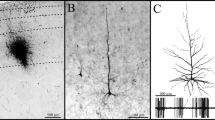
In Golgi material, the neurons of the periaqueductal gray matter (PAG) of the cat have been classified into five types, according to the following criteria: number of dendrites per cell, characteristics of secondary arborization, frequency of spines and axon caliber. Type 1 cells, which are multipolar and rich in spines are the most frequent, and are probably intranuclear neurons. Type 4 cells have a short axon which ends in the PAG, but they differ from Type 1 in that their dendritic ramification is of a different type and there are few spines. Type 2 and 3 neurons have a thick axon which runs outside the PAG, and dendrites rich in spines. Type 2 cells have more primary dendrites, while Type 3 neurons have dendrites which may spread outside the PAG. Type 5 cells have dendrites with few spines and no secondary ramification. Their thick and long axon projects outside the PAG. Type 2, 3 and 5 cells have been considered projective neurons. The various neuron types are present in every area of the PAG, although in the ventral region there is a predominance of Type 2 and 5 neurons, in the dorsal regions of Type 2 and 3 cells, and in lateral regions of Type 3 and 5 cells. Local intrinsic circuits have been observed in which both the interneurons and the projective, with early axonic collaterals, are involved. The prevalence of neurons to which an afferent role has been attributed (Type 2 and 3 cells) compared with efferent cells (Type 5), is in agreement with hodological studies which indicate that the PAG receives multiple and numerous afferents in comparison with the relatively scarce efferent fibers. These projections can be intensely and deeply elaborated and modulated by means of local intrinsic circuits.
Similar content being viewed by others
References
Barbaresi P, Conti F, Manzoni T (1982) Periaqueductal grey projection to the ventrobasal complex in the cat: a horse radish peroxidase study. Neurosci Lett 30: 205–209.
Basbaum AI, Fields HL (1984) Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci 7: 309–338.
Beitz AJ (1980) An analysis of regional subdivisions and neuronal populations in the rodent periaqueductal gray. Neuroscience Abst 6: 429.
Beitz AJ (1982) The organization of afferent projections to the midbrain periaqueductal gray of the rat. Neuroscience 7: 133–159.
Berman AL (1968) The brainstem of the cat. A cytoarchitectonic atlas with stereotaxic coordinates. The Univ of Wisconsin Press, Madison.
Bianchi R, Gioia M (1984) Ultrastructural features of the synapses of the periaqueductal gray matter (PAG) of the cat. J Hirnforsch 25: 275–284.
Bowker RM, Westlund KN, Coulter JD (1981) Serotonergic projections to the spinal cord from the midbrain in the rat: an immunocytochemical and retrograde study. Neurosci Lett 24: 221–226.
Cajal SR (1955) Histologie du système nerveux de l'homme et des vertébrés, Vol 1 and 2. Paris, Maloine, 1909. Reprinted: Madrid, Consejo Superior de Investigaciones Cientificas.
Carlton SM, Leichnetz GR, Young EG, Mayer DJ (1983) Supramedullary afferents of the nucleus raphe magnus in the rat. A study using the transcannula HRP gel and autoradiographic techniques. J Comp Neurol 214: 43–58.
Castaldi L (1923) Studi sulla struttura e sullo sviluppo del mesencefalo. Arch Ital Anat Embriol 20: 23–225.
Castiglioni AJ, Gallaway MC, Coulter JD (1978) Spinal projections from the midbrain in monkey. J Comp Neurol 178: 329–346.
Chan-Palay SL (1970) Interrelations of basket cell axons and climbing fibres in the cerebellar cortex of the rat. Z Anat Entwicklungsgesch 132: 191–227.
Chi CC (1970) An experimental silver study of the ascending projections of the central gray substance and adjacent tegmentum in the rat with observations in the cat. J Comp Neurol 139: 259–272.
Chung JM, Kevetter GA, Yezierski RP, Haber LH, Martin RF, Willis WD (1983) Midbrain nuclei projecting to the medial medulla oblungata in the monkey. J Comp Neurol 214: 93–102.
Cox W (1891) Imprägnation des zentralen Nervensystems mit Quecksilbersalzen. Arch Mikr Anat 37: 16–21.
Dalsass M, Kiser S, Mendershausen M, German DC (1981) Medial prefrontal cortical projections to the region of the dorsal periventricular catecholamine system. Neuroscience 6: 657–665.
Diamond J, Gray EG, Yasargil GM (1969) The function of dendritic spines: an hypothesis. J Physiol (Lond) 202: 116.
Diamond J, Gray EG, Yasargil GM (1970) The function of the dendrite spines: an hypothesis. In: Andersen P, Jansen JKS (eds). Universitetsforlaged Excitatory synaptic mechanisms, Oslo, pp 213–222.
Dostrovsky JO, Sessle BJ, Hu JW (1981) Presynaptic excitability changes produced in brainstem endings of tooth pulp afferents by raphe and other central and periperal influences. Brain Res 218: 141–160.
Dostrovsky JO, Shah Y, Gray BG (1983) Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus, and adjacent reticular formation. II. Effects on medullary dorsal horn nociceptive and non nociceptive neurons. J Neurophysiol 49: 948–960.
Eickhoff R, Handworker HO, McQueen DS, Schick E (1978) Noxious and tactile iput to medial structures of midbrain and pons in the rat. Pain 5: 99–113.
Gioia M, Tredici G, Bianchi R (1983) The ultrastructure of the periaqueductal gray matter of the cat. J Submicrosc Cytol 15: 1013–1026.
Gioia M, Bianchi R, Tredici G (1984) The cytoarchitecture of the periaqueductal gray matter (PAG) in the cat. A quantitative Nissl study. Acta Anatomica 199: 113–117.
Goldman PS, Nauta WJH (1976) Autoradiographic demonstration of a projection from prefrontal association cortex to the superior colliculus in the rhesus monkey. Brain Res 116: 145–149.
Gray BG, Dostrovsky JO (1983) Descending inhibitory influences from periaqueductal gray, nucleus raphe magnus and adjecent reticular formation. I. Effects on lumbar spinal cord nociceptive and nonnociceptive neurons. J Neurophysiol 49: 932–947.
Grofová I, Ottersen OP, Rinvik E (1978) Mesencephalic and diencephalic afferents to the superior colliculus and periaqueductal gray substance demonstrated by retrograde axonal transport of horseradish peroxidase in the cat. Brain Res 146: 205–220.
Hamilton BL (1973a) Cytoarchitectural subdivisions of the periaqueductal gray matter in the cat. J Comp Neurol 149: 1–28.
Hamilton BL (1973b) Projections of the nuclei of the periaqueductal gray matter in the cat. J Comp Neurol 152: 45–52.
Hamilton BL Skultety FM (1970) Efferent connections of the periaqueductal gray matter in the cat. J Comp Neurol 139: 105–114.
Hardy SGP, Leichnetz GR (1981a) Cortical projections to the periaqueductal gray in the monkey: a retrograde and orthograde horseradish peroxidase study. Neurosci Lett 22: 97–101.
Hardy SGP, Leichnetz GR (1981b) Frontal cortical projections to the periaqueductal gray in the rat: a retrograde and orthograde horseradish peroxidase study. Neurosci Lett 23: 13–17.
Hopkins DA, Holstege G (1978) Amygdaloid projections to the mesencephalon, pons and medulla oblungata in the cat. Exp Brain Res 32: 529–547.
Kerr FW (1975) The ventral spinothalamic tract and other ascending systems of the ventral funiculus ot the spinal cord. J Comp neurol 159: 335–356.
Kölliker A (1896) Handbuch der Gewerbelehre des Menschen. Verlag von Wilhem Engelmann, Leipzig.
Laemle LK (1979) Neuronal populations of the human periaqueductal gray, nucleus latelis. J Comp Neurol 186: 93–108.
Liu RP (1983) Laminar origin of spinal projection neurons to the periaqueductal gray of the rat. Brain Res 264: 118–122.
Liu RP., Hamilton BL (1980) Neurons of the periaqueductal gray matter as revealed by a Golgi study. J Comp Neurol 189: 403–418.
Llinás R, Hillman DE (1969) Physiological and morphological organization of the cerebellar circuits in various vertebrates. In: Llinás R (ed) Neurobiology of cerebellar evolution and development AMA-ERF Institute for Biomedical Research, Chicago, pp 43–73.
Mantyh PW (1982a) The Midbrain periaqueductal gray in the rat, cat and monkey: a Nissl, Weil, and Golgi analysis. J Comp Neurol 204: 349–363.
Mantyh PW (1982b) Forebrain projections to the periaqueductal gray in the monkey, with observation in the cat and rat. J Comp Neurol 206: 146–158.
Mantyh PW (1982c) The ascending input to the midbrain periaqueductal gray of the primate. J Comp Neurol 211: 50–64.
Mantyh PW, Peschanski M (1982) Spinal projections from the periaqueductal grey and dorsal raphe in the rat, cat and monkey. Neuroscience 7: 2769–2776.
Mayer ML (1982) Periaqueductal grey neuronal activity: correlation with EEG arousal evoked by noxious stimuli in the rat. Neurosci Lett 28: 297–301.
Mehler WR, Feferman ME, Nauta WJH (1960) Ascending axon degeneration following anterolateral cordotomy. An experimental study in the monkey. Brain 83: 718–750.
Menetrey D, Chaouch A, Binder D, Besson JM (1982) The origin of the spinomesencephalic tract in the rat: an anatomical study using the retrograde transport of horseradish peroxidase. J Comp Neurol 206: 193–207.
Nauta WJH (1958) Hippocampal projections and related neural pathways to the mid-brain in the cat. Brain 81: 319–341.
Oliveras JL, Woda A, Guilbaud G, Besson JM (1974) Inhibition of the jaw opening reflex by electrical stimulation of the periaqueductal gray matter in the awake, unrestrained cat. Brain Res 72: 328–331.
Olszewski J, Baxter D (1954) Cytoarchitecture of the human brainstem. JB Lippincott Co, Philadelphia.
Palay SL, Chan-Palay V (1974) Cerebellar cortex, cytology and organization. Springer, Berlin Heidelberg New York.
Parent A, Steriade M (1981) Afferents from the periaqueductal gray, medial hypothalamus and medial thalamus to the midbrain reticular core. Brain Res Bull 7: 411–418.
Peters A, Kaiserman-Abramof IR (1970) The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am J Anat 127: 321–356.
Prichard SM, Beitz AJ (1980) A Golgi analysis of the rodent periaqueductal gray. Neuroscience Abst 6: 429.
Ramon-Moliner E (1962) An attempt at classifying nerve cells on the basis of their dendritic patterns. J Comp Neurol 119: 211–227.
Reynolds DV (1969) Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science 164: 444–445.
Sessle BJ, Hu JW, Dubner R, Lucier GE (1981) Functional properties of neurons in cat trigeminal subnucleus caudalis (medullary dorsal horn). II. Modulation of responses to noxious and non noxious stimuly by periaqueductal gray, nucleus raphe magnus, cerebral cortex and afferent influences, and effect of naloxone. J Neurophysiol 45: 193–207.
Taber E (1961) The cytoarchitecture of the brainstem of the cat. I. Brainstem nuclei of cat. J Comp Neurol 116: 27–69.
Tredici G, Bianchi R, Gioia M (1983) Short intrinsic circuit in the periaqueductal gray matter of the cat. Neurosci Lett 39: 131–136.
Tredici G, Gioia M, Bianchi R, Barajon I (1984) Cortical projection to the mesencepahlic periaqueductal gray matter of the rat. Neurosci Lett 18S: 250.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gioia, M., Tredici, G. & Bianchi, R. A golgi study of the periaqueductal gray matter in the cat. Neuronal types and their distribution. Exp Brain Res 58, 318–332 (1985). https://doi.org/10.1007/BF00235313
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00235313