Summary
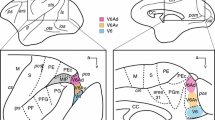
Physiological and anatomical criteria were used to clearly establish the existence of a pretectal relay of visual information to the ipsilateral inferior olive in the macaque monkey. After injection of horseradish peroxidase into the inferior olivary nucleus, retrogradely labelled neurons were found in the nucleus of the optic tract (NOT) and the dorsal terminal nucleus of the accessory optic tract (DTN). The labelled cells were distributed in a sparse band arching below the margin of the brachium of the superior colliculus between the dorsal and lateral borders of the brainstem at the caudal edge of the pulvinar. Various types of cells could be distinguished. More superficially the cells were extremely spindle shaped, cells deeper within the midbrain had more compact somata. NOT-DTN neurons in the same region were also found to respond with short latencies to electrical stimulation of both the inferior olive and the optic chiasm. All neurons in the NOTDTN which were antidromically activated from the inferior olive were also found to have direction specific binocular visual responses. Such neurons were excited by ipsiversive motion and suppressed by contraversive motion, regardless of whether large area random dot stimuli moved across the visual field or small single dots moved across the fovea. Direct retinal input to these neurons was via slowly conducting fibers (3–9 m/s) from the monkey's optic tract conduction velocity spectrum. As shown previously for non-primates, NOT-DTN cells may also in the monkey carry a signal representing the velocity error between stimulus and retina (retinal slip), and relay this signal into the circuitry mediating the optokinetic reflex.
Similar content being viewed by others
References
Ballas I, Hoffmann K-P (1985) A correlation between receptive field properties and morphological structures in the pretectum of the cat. J Comp Neurol 238: 417–428
Ballas I, Hoffmann K-P, Wagner HJ (1981) Retinal projection to the nucleus of the optic tract in the cat as revealed by retrograde transport of horseradish peroxidase. Neurosci Lett 26: 197–202
Benevento LA (1975) Stereotaxic coordinates for the rhesus monkey thalamus and mesencephalon referencing visual afferents and cytoarchitecture. J Hirnforsch 16: 117–129
Bishop GH, Clare MH, Landau WM (1969) Further analysis of fibre groups in the optic tract of the cat. Exp Neurol 24: 386–399
Cazin L, Precht W, Lannou J (1980) Pathways mediating optokinetic responses of vestibular nucleus neurons in the rat. Pflügers Arch 384: 19–29
Cazin L, Lannou J, Precht W (1984) An electrophysiological study of pathways mediating optokinetic responses to the vestibular nucleus in the rat. Exp Brain Res 54: 337–348
Cheng M, Outerbridge JM (1975) Optokinetic nystagmus during selective retinal stimulation. Exp Brain Res 23: 129–139
Cochran SL, Dieringer N, Precht W (1984) Basic optokineticocular reflex pathways in the frog. J Neurosci 4: 43–57
Collewijn H (1975a) Oculomotor areas in the rabbit's midbrain and pretectum. J Neurobiol 6: 3–22
Collewijn H (1975b) Direction-selective units in the rabbit's nucleus of the optic tract. Brain Res 100: 489–508
Collewijn H, Holstege G (1984) Effects of neonatal and late unilateral enucleation on optokinetic responses and optic nerve projections in the rabbit. Exp Brain Res 57: 138–150
Cooper HM, Magnin M (1986) A common mammalian plan of accessory optic system organization revealed in all primates. Nature 324: 457–459
Cynader M, Berman N (1972) Receptive-field organization of monkey superior colliculus. J Neurophysiol 35: 187–201
Dubois MFW, Collewijn H (1979) Optokinetic reactions in man elicited by localized retinal motion stimuli. Vision Res 19: 1105–1115
Eccles JC, Ito M, Szentágothai J (1967) The cerebellum as a neuronal machine. Springer, Berlin Heidelberg New York, p 175
Farmer SG, Rodieck RW (1982) Ganglion cells of the cat accessory optic system: morphology and retinal topography. J Comp Neurol 205: 190–198
Fite KV, Reiner A, Hunt SP (1979) Optokinetic nystagmus and the accessory optic system of pigeon and turtle. Brain Behav Evol 16: 192–202
Fite KV, Montgomery N (1982) Neural correlates of optokinetic nystagmus (OKN) in the amphibian mesencephalon: a functional analysis. Neurosci 7: 569–570
Gioanni H, Rey J, Villalobos J, Richard D, Dalbera A (1983) Optokinetic nystagmus in the pigeon (Columba livia). II. Role of the pretectal nucleus of the accessory optic system (AOS). Exp Brain Res 50: 237–247
Giolli RA (1963) An experimental study of the accessory optic system in the cynomolgus monkey. J Comp Neurol 121: 89–108
Grasse KL, Cynader MS (1984) Electrophysiology of lateral and dorsal terminal nuclei of the cat accessory optic system. J Neurophysiol 51: 276–293
Grasse KL, Cynader MS (1986) Response properties of single units in the accessory optic system of the dark reared cat. Dev Brain Res 27: 199–210
Hendrickson A, Wilson ME, Toyne MJ (1970) The distribution of optic nerve fibers in Macaca mulatta. Brain Res 23: 425–427
Hoffmann K-P (1973) Conduction velocity in pathways from retina to superior colliculus in the cat: a correlation with receptivefield properties. J Neurophysiol 36: 409–424
Hoffmann K-P (1983) Effects of early monocular deprivation on visual input to cat nucleus of the optic tract. Exp Brain Res 51: 236–246
Hoffmann K-P (1985) Direction selective cells in the nucleus of the optic tract in the squirrel monkey. Neurosci Abstr 11: 1009
Hoffmann K-P (1986) Visual inputs relevant for the optokinetic nystagmus in mammals. In: Freund HJ, Büttner U, Cohen B, Noth J (eds) Progress in Brain Research, Vol 64: 75–84
Hoffmann K-P, Behrend K, Schoppmann A (1976) A direct afferent visual pathway from the nucleus of the optic tract to the inferior olive in the cat. Brain Res 115: 150–153
Hoffmann K-P, Distler C (1986) The role of direction selective cells in the nucleus of the optic tract of cat and monkey during optokinetic nystagmus. In: Keller EL, Zee DS (eds) Adaptive processes in visual and oculomotor systems. Pergamon Press, Oxford, pp 261–266
Hoffmann K-P, Schoppmann A (1981) A quantitative analysis of the direction-specific response of neurons in the cat's nucleus of the optic tract. Exp Brain Res 42: 146–157
Hoffmann K-P, Stone J (1985) Retinal input to the nucleus of the optic tract of the cat assessed by antidromic activation of ganglion cells. Exp Brain Res 59: 395–403
Horn AKE, Hoffmann K-P (1987) Combined GABA-immunohistochemistry and TMB-HRP histochemistry of pretectal nuclei projecting to the inferior olive in rats, cats, and monkeys. Brain Res 409: 133–138
Hutchins R, Weber JT (1985) The pretectal complex of the monkey: a reinvestigation of the morphology and retinal terminations. J Comp Neurol 232: 425–442
Itaya SK, Van Hoesen GW (1983) Retinal axons to the medial terminal nucleus of the accessory optic system in old world monkeys. Brain Res 269: 361–364
Ito M, Nisimaru N, Yamamoto M (1977) Specific patterns of neuronal connexions involved in the control of the rabbit's vestibuloocular reflexes by the cerebellar flocculus. J Physiol 265: 833–854
Kato I, Harada K, Hasegawa T, Igarashi T, Koike Y, Kawasaki T (1986) Role of the nucleus of the optic tract in monkeys in relation to optokinetic nystagmus. Brain Res 364: 12–22
Koerner F, Schiller PH (1972) The optokinetic response under open and closed loop conditions in the monkey. Exp Brain Res 14: 318–330
Lazar G, Alkonyi B, Toth P (1983) Re-investigation of the role of the accessory optic system and pretectum in the horizontal optokinetic head nystagmus of the frog. Lesion experiments. Acta Biol Hung 34: 385–393
Lin H, Giolli RA (1979) Accessory optic system of rhesus monkey. Exp Neurol 63: 163–176
Lisberger SG, Miles FA, Zee DS (1984) Signals used to compute errors in monkey vestibuloocular reflex: possible role of flocculus. J Neurophysiol 52: 1140–1153
Maekawa K, Simpson JI (1973) Climbing fiber responses evoked in vestibulocerebellum of rabbit from visual system. J Neurophysiol 36: 649–666
Maekawa K, Takeda T (1979) Origin of descending afferents to the rostral part of dorsal cap of inferior olive which transfers contralateral optic activities to the flocculus. An horseradish peroxidase study. Brain Res 172: 393–405
Maekawa K, Takeda T, Kimura M (1984) Responses of the nucleus of the optic tract neurons projecting to the nucleus reticularis tegmenti pontis upon optokinetic stimulation in the rabbit. Neurosci Res 2: 1–26
Magnin M, Courjon JH, Flandrin JM (1983) Possible visual pathways to the cat vestibular nuclei involving the nucleus prepositus hypoglossi. Exp Brain Res 51: 298–303
Manteuffel G, Petersen J, Himstedt W (1983) Optic nystagmus and nystagmogen centers in the European fire salamander. Zool Jb Physiol 87: 113–125
Mesulam MM (1978) Tetramethylbetlzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualization of neuron afferents and efferents. J Histochem Cytochem 26: 123–131
Miyashita M (1986) Neuronal circuit modifications underlying adaptation of the vestibuloocular reflex. In: Keller EL, Zee DS (eds) Adaptive processes in visual and oculomotor systems. Pergamon Press, Oxford, pp 435–442
de Monasterio FM (1978) Properties of ganglion cells with atypical receptive-field organization in retina of macaques. J Neurophysiol 41: 1435–1449
Oyster CW, Takahashi E, Collewijn H (1972) Direction-selective retinal ganglion cells and control of optokinetic nystagmus in the rabbit. Vision Res 12: 183–193
Pasik T, Pasik P (1964) Optokinetic nystagmus: an unlearned response altered by section of chiasma and corpus callosum in monkeys. Nature 203: 609–611
Perry VH, Cowey A (1984) Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience 12: 1125–1137
Precht W, Strata P (1980) On the pathway mediating optokinetic responses in vestibular nuclear neurons. Neuroscience 5: 777–787
Sanchez RM, Dunkelberger GR, Quigley HA (1986) The number and diameter distribution of axons in the monkey optic nerve. Invest Ophthalmol Vis Sci 27: 1342–1350
Scalia F (1972) The termination of retinal axons in the pretectal region of mammals. J Comp Neurol 145: 223–258
Schiff D, Cohen B, Raphan T (1987) Nystagmus induced by stimulation of the nucleus of the optic tract in the monkey (in press)
Schiller PH, Malpeli JG (1977) Properties and tectal projections of monkey retinal ganglion cells. J Neurophysiol 40: 428–445
Schoppmann A, Hoffmann K-P (1976) Continuous mapping of direction selectivity in the cat's visual cortex. Neurosci Lett 2: 177–181
Sekiya H, Kawamura K (1985) An HRP study in the monkey of olivary projections from the mesodiencephalic structures with particular reference to pretecto-olivary neurons. Arch Ital Biol 123: 171–183
Simpson JI (1984) The accessory optic system. Ann Rev Neurosci 7: 13–41
Smith OA, Kastella KG, Randall DC (1972) A stereotaxic atlas of the brainstem of Macaca mulatta in the sitting position. J Comp Neurol 145: 1–24
Snider RS, Lee JL (1961) A stereotaxic atlas of the monkey brain (Macaca mulatta). The University of Chicago Press, Chicago
Szabo J, Cowan WM (1984) A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis). J Comp Neurol 222: 265–300
Terasawa K, Otani K, Yanada Y (1979) Descending pathways of the nucleus of the optic tract in the rat. Brain Res 173: 405–418
Walberg F, Nordby T, Hoffmann K-P, Holländer H (1981) Olivary afferents from the pretectal nuclei in the cat. Anat Embryol 161: 291–304
Weber JT (1985) Pretectal complex and accessory optic system in primates. Brain Behav Evol 26: 117–140
Westheimer G, Blair SM (1974) Unit activity in accessory optic system in alert monkeys. Invest Ophthalmol 13: 533–534
Zee DS, Tusa RJ, Herdman SJ, Butler PH, Guecer G (1986) The acute and chronic effects of bilateral occipital lobectomy upon eye movements in monkey. In: Keller EL, Zee DS (eds) Adaptive processes in visual and oculomotor systems. Pergamon Press, Oxford, pp 267–274
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hoffmann, K.P., Distler, C., Erickson, R.G. et al. Physiological and anatomical identification of the nucleus of the optic tract and dorsal terminal nucleus of the accessory optic tract in monkeys. Exp Brain Res 69, 635–644 (1988). https://doi.org/10.1007/BF00247315
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00247315