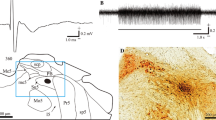
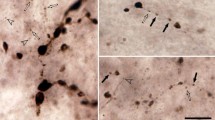
Summary
Single units were recorded, using extra-cellular glass microelectrodes, in the ventrobasal complex of the thalamus of rats under halothane-nitrous oxide anaesthesia. The animals had previously undergone a large bilateral section of the trigeminal sensory complex just above the obex to deprive the caudal part of the trigeminal sensory complex (subnucleus caudalis) of its trigeminal afferents. As observed on frontal slices our lesions impaired the whole descending tract and, in most cases, the intratrigeminal pathways between the rostral and the caudal part of the complex. Forty-seven units responding to a somatic mechanical noxious stimulation applied to the trigeminal area were recorded in these conditions. Forty-two of these had a receptive field (or at least a part of it) in or around the oral and nasal cavities, and 5 in the peripheral part of the face. These data confirm the hypothesis that the rostral part of the trigeminal sensory complex participates in pain sensory pathways, as a first relay site between nociceptive primary afferents coming from oral, perioral and perinasal areas, and the ventrobasal complex of the thalamus. In addition, they suggest that the intra-trigeminal pathways are not essential for the transmission of these nociceptive inputs, to the lateral thalamus.
Similar content being viewed by others
References
Albe-Fessard D, Stutinsky F, Libouban F (1971) Atlas stéréotaxique du diencéphale du rat blanc. CNRS, Paris
Arvidsson J, Gobel S (1981) An HRP study of the central projections of primary trigeminal neurons which innervate tooth pulp in the cat. Brain Res 208: 1–16
Azerad J, Woda A (1976) Tooth pulp projection to the trigeminal complex and jaw opening reflex in the cat. J Biol Bucc 4: 109–115
Azerad J, Woda A, Albe-Fessard D (1977) Identification dans les enregistrements par microélectrodes de verre des activités recueillies au voisinage des axones et des corps cellulaires du complexe sensitif trigéminal. CR Acad Sc 285: 797–800
Azerad J, Woda A, Albe-Fessard D (1982) Physiological properties of neurons in different parts of the cat trigeminal sensory complex. Brain Res 246: 7–21
Benoist JM, Kayser V, Gautron M, Guilbaud G (1983) Low dose of morphine strongly depresses responses of specific nociceptive neurones in the ventrobasal complex of the rat. Pain 15: 333–344
Broton JG, Rosenfeld JP (1985) Effects of trigeminal tractotomy on facial thermal nociception in the rat. Brain Res 333: 63–72
Bruce LL, Mc Haffie JG, Stein BE (1987) The organization of trigeminotectal and trigeminothalamic neurons in rodents: a double-labeling study with fluorescent dyes. J Comp Neurol 262: 315–330
Burton H, Craig AD (1979) Distribution of trigeminothalamic projection cells in cat and monkey. Brain Res 161: 515–521
Burton H, Craig AD Jr, Poulos DA, Molt JT (1979) Efferent projections from temperature sensitive recording loci within the marginal zone of the nucleus caudalis of the spinal trigeminal complex in the cat. J Comp Neurol 183: 753–778
Clarke WB, Bowsher D (1962) Terminal distribution of primary afferent trigeminal fibers in the rat. Exp Neurol 6: 372–383
Clarke RW (1984) The trigeminal nuclei in pain. In: Holden AB, Winlow W (ed) The neurobiology of pain. Manchester University Press, Manchester, pp 70–81
Dallel R, Raboisson P, Auroy P, Woda A (1988) The rostral part of the trigeminal sensory complex is involved in orofacial nociception. Brain Res 448: 7–19
Dubner R, Sessle BJ, Storey AT (1978) The neural basis of oral and facial function. Plenum, New York
Eisenman J, Landgren S, Novin D (1963) Functionnal organization in the main sensory trigeminal nucleus and in the rostral subdivision of the nucleus of the spinal trigeminal tract in the cat. Acta Physiol Scand 59: 1–44
Erzurumlu RS, Killackey HP (1980) Diencephalic projections of the subnucleus interpolaris of the brainstem trigeminal complex in the rat. Neuroscience 5: 1891–1902
Fukushima T, Kerr FWL (1979) Organization of trigemino-thalamic tracts and other thalamic afferent systems of the brainstem in the rat: presence of gelatinosa neurons with thalamic connections. J Comp Neurol 183: 169–184
Graham SH, Sharp FR, Dillon W (1988) Intraoral sensation in patients with brainstem lesions: role of the rostral spinal trigeminal nuclei in pons. Neurology 38: 1529–1533
Grant FC, Groff RA, Lewy FH (1940) Section of the descending spinal root of the fifth cranial nerve. Arch Neurol Psychiat 43: 498–509
Greenwood LF, Sessle BJ (1976) Inputs to trigeminal brain stem neurones from facial, oral, tooth plup and pharyngolaryngeal tissues. II. Role of trigeminal nucleus caudalis in modulating responses to innocuous and noxious stimuli. Brain Res 117: 227–238
Guilbaud G, Peschanski M, Gautron M, Binder D (1980) Neurons responding to noxious stimulation in V.B. complex and caudal adjacent regions in the thalamus of the rat. Pain 8: 308–318
Hayashi H, Sumino R, Sessle BJ (1984) Functionnal organization of trigeminal subnucleus interpolaris: nociceptive and innocuous afferent inputs, projections to thalamus, cerebellum, and spinal cord, and descending modulation from periaqueductal gray. J Neurophysiol 51: 890–905
Hockfield S, Gobel S (1982) An anatomical demonstration of projections to the medullary dorsal horn (trigeminal nucleus caudalis) from rostral trigeminal nuclei and the contralateral caudal medulla. Brain Res 252: 203–211
Hu JW, Sessle BJ (1984) Comparison of responses of cutaneous nociceptive and nonnociceptive brain stem neurons in trigeminal subnucleus caudalis (medullary dorsal horn) and subnucleus oralis to natural and electrical stimulation of tooth pulp. J Neurophysiol 52: 39–53
Ikeda M, Tanami T, Matsushita M (1984) Ascending and descending internuclear connections of the trigeminal sensory nuclei in the cat: a study with the retrograde and anterograde horseradish peroxidase technique. Neuroscience 4: 1243–1260
Jacquin MF, Mooney RD, Rhoades RW (1986) Morphology, response properties, and collateral projections of trigemino-thalamic neurons in brainstem subnucleus interpolaris of rat. Exp Brain Res 61: 457–468
Marfurt CF, Turner DF (1984) The Central projections of tooth pulp afferent neurons in the rat as determined by the transganglionic transport of horseradish peroxydase. J Comp Neurol 223: 535–547
Matsushita M, Ikeda M, Okado N (1982) The cells of origin of the trigeminothalamic, trigeminospinal and trigeminocerebellar projections in the cat. Neuroscience 7: 1439–1454
Nasution ID, Shigenaga Y (1987) Ascending and descending internuclear projections within the trigeminal sensory nuclear complex. Exp Brain Res 425: 234–247
Nord SG (1976) Responses of neurons in rostral and caudal trigeminal nuclei to tooth pulp stimulation. Brain Res Bull 1:489–492
Peschanski M (1984) Trigeminal afferents to the diencephalon in the rat. Neuroscience 12: 465–487
Sessle BJ, Greenwood LF (1976) Inputs to trigeminal brain stem neurones from facial, oral, tooth pulp and pharyngo laryngeal tissues. I. Responses to innocuous and noxious stimuli. Brain Res 117: 211–226
Shigenaga Y, Nakatani Z, Nishimori T, Suemune S, Kuroda R, Matano S (1983) The cells of origin of cat trigeminothalamic projections: especially in the caudal medulla. Brain Res 277: 201–222
Shigenaga Y, Okamoto T, Nishimori T, Suemune S, Nasution ID, Chen IC, Tsuru K, Yoshida A, Tabuchi K, Hosoi M, Tsuru H (1986a) Oral and facial representation in the trigeminal principal and rostral spinal nuclei of the cat. J Comp Neurol 244: 1–18
Shigenaga Y, Suemune S, Nishimura M, Nishimori T, Sato H, Ishidori H, Yoshida A, Tsuru K, Tsuiki Y, Dateoka Y, Nasution ID, Hosoi M (1986b) Topographic representation of lower and upper teeth within the trigeminal sensory nuclei of adult cat as demonstrated by the transganglionic transport of horseradish peroxidase. J Comp Neurol 251: 299–316
Vyklicky L, Keller D (1973) Central projection of tooth pulp primary afferents in the cat. Acta Neurobiol Exp 33: 803–809
Vyklicky L, Keller O, Jastreboff P, Vyklicky L Jr, Butkhuzi SM (1977) Spinal trigeminal tractotomy and nociceptive reactions evoked by tooth pulp stimulation in the cat. J Physiol 73: 379–386
Woda A, Azerad J, Albe-Fessard D (1977) Mapping of the trigeminal sensory complex of the cat: characterization of its neurons by stimulations of peripheral fields, dental pulp afferents and thalamic projections. J Physiol 73: 367–378
Young RF (1982) Effects of trigeminal tractotomy on dental sensation in humans. J Neurosurg 56: 812–818
Young RF, King RB (1972) Excitability changes in trigeminal primary afferent fibers in response to noxious and non-noxious stimuli. J Neurophysiol 35: 87–95
Young RF, Perryman KM (1984) Pathways for orofacial pain sensation in the trigeminal brain stem nuclear complex of the macaque monkey. J Neurosurg 61: 563–568
Young RF, Perryman KM (1986) Neuronal responses in rostral trigeminal brain-stem nuclei of macaque monkeys after chronic trigeminal tractotomy. J Neurosurg 65: 508–516
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raboisson, P., Dallel, R. & Woda, A. Responses of neurones in the ventrobasal complex of the thalamus to orofacial noxious stimulation after large trigeminal tractotomy. Exp Brain Res 77, 569–576 (1989). https://doi.org/10.1007/BF00249609
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00249609