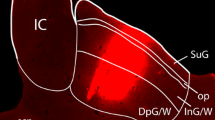
Summary
In the course of our study on the neuronal connections of the subparafascicular nucleus (SPF) in the rat, descending projections from the SPF to the lower brain stem were examined by using the anterograde tracer PHA-L (Phaseolus vulgaris leukoagglutinin) and retrograde tracer WGA-HRP (horseradish peroxidase conjugated to wheat germ agglutinin). When PHA-L was injected into the magnocellular and/or parvicellular division of the SPF (SPFm and/or SPFp), presumed terminal labeling was seen, bilaterally with an ipsilateral dominance, in the mesencephalic and pontine central gray matter, peripheral shell regions of the inferior colliculus, cuneiform nucleus, and superior olivary complex (mainly in the superior paraolivary nucleus, and additionally in the nuclei of the trapezoid body). A few labeled axon terminals were also seen in the cochlear nuclei bilaterally with a contralateral dominance. In the second set of experiments, WGA-HRP was injected into the inferior colliculus, superior olivary complex, or cochlear nuclei. When WGA-HRP was injected into the peripheral shell regions of the inferior colliculus or the superior olivary complex, many labeled neuronal cell bodies were seen in the SPFm bilaterally with an ipsilateral dominance, and a moderate number of labeled neuronal cell bodies were observed in the SPFp (lateral SPF) bilaterally with an ipsilateral dominance. When WGA-HRP was injected into the cochlear nuclei, a moderate number of labeled neuronal cell bodies were observed in the SPFm and SPFp bilaterally with a contralateral dominance. The results indicate that the SPFm and SPFp (lateral SPF) of the rat send a considerable number of projection fibers to the lower brain stem. The target regions of these projection fibers include the auditory relay nuclei, such as the inferior colliculus, superior olivary complex, and cochlear nuclei.
Similar content being viewed by others
References
Aitkin LM, Kenyon CE, Philpott P (1981) The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. J Comp Neurol 196: 25–40
Bentivoglio M, Molinari M (1984) The interrelations between cell groups in the caudal diencephalon of the rat projecting to the striatum and to the medulla oblongata. Exp Brain Res 54: 57–65
Berkley KJ, Hand PJ (1978) Efferent projections of the gracile nucleus in the cat. Brain Res 153: 263–283
Berkley KJ, Budell RJ, Blomqvist A, Bull M (1986) Output systems of the dorsal column nuclei in the cat. Brain Res Rev 11: 199–225
Burton H, Craig AD (1983) Spinothalamic projections in cat, raccoon and monkey: a study based on anterograde transport of horseradish peroxidase. In: Macci G, Rustioni A, Spreafico R (eds) Somatosensory integration in the thalamus. Elsevier, Amsterdam New York, pp 17–41
Calford MB (1983) The parcellation of the medial geniculate body of the cat defined by the auditory response properties of single units. J Neurophysiol 3: 2350–2364
Calford MB, Aitkin LM (1983) Ascending projections to the medial geniculate body of the cat: evidence for multiple, parallel auditory pathways through the thalamus. J Neurosci 3: 2365–2380
Casseday JH, Diamond IT, Harting JK (1976) Auditory pathways to the cortex in Tupaia glis. J Comp Neurol 116: 303–340
Cintas HM, Rutherford JG, Gwyn DG (1980) Some midbrain and diencephalic projections to the inferior olive in the rat. In: Courville J et al. (eds) The inferior olivary nucleus: anatomy and physiology. Raven Press, New York, pp 73–96
Erulkar SD (1975) Physiological studies of the inferior colliculus and medial geniculate complex. In: Keidel WD, Neff WD (eds) Handbook of sensory physiology, vol II/2. Springer, Berlin Heidelberg New York, pp 145–198
Faul RLM, Mehler WR (1985) Thalamus. In: Paxinos G (eds) The rat nervous system, vol I. Forebrain and midbrain. Academic Press, Sydney, pp 129–168
Gerfen CR, Sawchenko PE (1984) An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunnohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res 290: 219–238
Henkel CK, Linauts M, Martin GF (1975) The origin of the annuro-olivary tract with notes on other mesencephalo-olivary pathways. A study by the horseradish peroxidase method. Brain Res 100: 145–150
Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoporoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 29: 577–580
Itoh K, Kaneko T, Kudo M, Mizuno N (1984) The intercollicular region in the cat: a possible relay in the parallel somatosensory pathways from the dorsal column nuclei to the posterior complex of the thalamus. Brain Res 308: 166–171
LeDoux JE, Ruggiero DA, Reis DJ (1985) Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J Comp Neurol 242: 182–213
LeDoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ (1987) Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol 264: 123–146
Mehler WR (1980) Subcortical afferent connections of the amygdala in the monkey. J Comp Neurol 190: 733–762
Mehler WR, Pretorius JK, Phelan KD, Mantyh PW (1981) Diencephalic afferent connections of the amygdala in the squirrel monkey with observations and comments on the cat and rat. In: Ben-Ari Y (eds) The amygdaloid complex. INSERM Symposium No. 20. Elsevier, Amsterdam, pp 105–120
Mesulam M-M (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-cartinogenic blue reaction-product with superior sensitivity for visualizing neuronal afférents and efferents. J Histochem Cytochem 26: 106–117
Moore RY, Goldberg JM (1963) Ascending projections of the inferior colliculus in the cat. J Comp Neurol 121: 109–136
Moore RY, Goldberg JM (1966) Projections of the inferior colliculus in the monkey. Exp Neurol 14: 429–438
Moriizumi T, Hattori T (1991) Non-dopaminergic projection from the subparafascicular area to the temporal cortex in the rat. Neurosci Lett 129: 127–130
Oliver DL, Hall WC (1978) The medial geniculate body of the tree shrew, Tupaia glis. I. Cytoarchitecture and midbrain connections. J Comp Neurol 182: 423–458
Ottersen OP, Ben-Ari Y (1979) Afferent connections to the amygdaloid complex of the rat and cat. I. Projections from the thalamus. J Comp Neurol 187: 401–424
Paxinos G, Watson C (1986) The rat brain in stereotaxic coordinates. Academic Press, Sydney
Peschanski M, Mantyh PW (1983) Efferent connections of the subparafascicular area of the mesodiencephalic junction and its possible involvment in stimulation-produced analgesia. Brain Res 263: 181–190
Russchen FT (1982) Amygdalopetal projections in the cat. II. Subcortical afferent connections. A study with retrograde tracing techniques. J Comp Neurol 207: 157–176
Saint-Cyr JA, Courville J (1981) Sources of descending afferents to the inferior olive from upper brain stem in the cat as revealed by the retrograde transport of horseradish peroxidase. J Comp Neurol 198: 567–581
Schroeder DM, Jane JA (1971) Projections of dorsal column nuclei and spinal cord to brain stem and thalamus in the tree shrew, Tupaia glis. J Comp Neurol 142: 309–350
Takada M, Li ZK, Hattori T (1988) Single thalamic dopaminergic neurons project to both the neocortex and spinal cord. Brain Res 455: 346–352
Turner BH, Herkenham M (1991) Thalamoamygdaloid projections in the rat: a test of the amygdala's role in sensory processing. J Comp Neurol 313: 295–325
Webster WR, Aitkin LM (1975) Central auditory processing. In: Gazzaniga MS, Blakemore C (eds) Handbook of physiology. Academic Press, New York pp 325–364
Wiberg M, Blomqvist A (1984) The projection to the mesencephalon from the dorsal column nuclei: an anatomical study in the cat. Brain Res 311: 225–244
Yasui Y, Saper CB, Cechetto DF (1989) Calcitonin gene-related peptide immunoreactivity in the visceral sensory cortex, thalamus, and related pathways in rat. J Comp Neurol 290: 487–501
Yasui Y, Kayahara T, Nakano K, Mizuno N (1990) The subparafascicular thalamic nucleus of the rat receives projection fibers from the inferior colliculus and auditory cortex. Brain Res 537: 323–327
Yasui Y, Saper CB, Cechetto DF (1991) Calcitonin gene-related peptide (CGRP) immunoreactive projections from the thalamus to the striatum and amygdala in the rat. J Comp Neurol 308: 293–310
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yasui, Y., Nakano, K. & Mizuno, N. Descending projections from the subparafascicular thalamic nucleus to the lower brain stem in the rat. Exp Brain Res 90, 508–518 (1992). https://doi.org/10.1007/BF00230933
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230933