Abstract
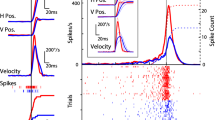
We investigated the relationships between cortical arousal and cholinergic facilitation of evoked responses in the auditory cortex. The basal forebrain (BF) was stimulated unilaterally, while cluster recordings were obtained simultaneously from both auditory cortices in urethane-anesthetized rats. The global electroencephalogram (EEG; large frontoparietal derivation) and the local EEG (from the auditory cortex) were recorded. The BF was stimulated at two intensities, a lower one which did not desynchronize the EEG and a higher one which did. Twenty pairing trials were delivered, during which a tone was presented 50 ms after the end of the BF stimulation. At low intensity, the pairing procedure led to a transient increase in the ipsilateral tone-evoked responses. At high intensity, the pairing increased the ipsilateral evoked responses up to 15 min after pairing. Such effects were not observed for the contralateral recordings. Systemic atropine injection prevented the facilitations observed ipsilaterally. BF stimulations alone did not induce any increased evoked response either at low or at high intensity. These results show (1) that a tone, presented while the cortex is activated by cholinergic neurons of the BF, evokes enhanced cortical responses, and (2) that the duration of this facilitation is dependent on the stimulation intensity. These results are discussed in the context of neural mechanisms involved in general arousal and cortical plasticity.
Similar content being viewed by others
References
Ashe JH, McKenna TM, Weinberger NM (1989) Cholinergic modulation of frequency receptive fields in auditory cortex II. Frequency-specific effects of anticholinesterases provide evidence for a modulatory action of endogenous ACh. Synapse 4(1): 44–54
Ashe JH, Weinberger NM (1991) Acetylcholine modulation of cellular excitability via muscarinic receptors: functional plasticity in auditory cortex. In Richardson RT (ed) Activation to acquisition: functional aspects of the basal forebrain cholinergic system. Birkhauser, Boston, pp. 189–246
Bakin JS, Weinberger NM (1990) Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res 536:271–286
Beaulieu C, Somogyi P (1991) Enrichment of cholinergic synaptic terminals on GABAergic neurons and coexistence of immunoreactive GABA and choline actetyltransferase in the same synaptic terminals in the striate cortex of the cat. J Comp Neurol 304:666–680
Belardetti F, Borgia R, Mancia M (1977) Prosencephalic mechanisms of EEG desynchronization in cerveau isolé cats. Electroencephalogr Clin Neurophysiol 42:213–225
Bigl V, Woolf NJ, Butcher LL (1982) Cholinergic projections from the basal forebrain to frontal, parietal, temporal, occipital and cingulate cortices: a combined fluorescent tracer and acetylcholinesterase analysis. Brain Res Bull 8(6): 727–749
Brugge JF, Merzenich MM (1973) Responses of neurons in auditory cortex of macaque monkey to monaural and binaural stimulation. J Neurophysiol 36:1130–1158
Buzsàki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH (1988) Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci 8(11): 4007–4026
Casamenti F, Deffenu G, Abbamondi AL, Pepeu G (1986) Changes in cortical acetylcholine output induced by modulation of the nucleus basalis. Brain Res Bull 16:689–695
Celesia GG, Jasper HH (1966) Acetylcholine release from cerebral cortex in relation to state of activation. Neurology 16:1053–1064
Clarey JC, Irvine DRF (1990) The anterior ectosylvian sulcal auditory field in the cat. I. An electrophysiological study of its relationship to surrounding auditory cortical fields. J Comp Neurol 301:289–303
Cox CL, Metherate R, Weinberger NM, Ashe JH (1992) Synaptic potentials and amino acid antagonists in auditory cortex. Brain Res Bull 28:401–410
Detari L, Vanderwolf CH (1987) Activity of identified cortically projecting and other basal forebrain neurons during slow-waves and cortical activation in anaesthetized rats. Brain Res 437:1–8
Diamond DM, Weinberger NM (1986) Classical conditioning rapidly induces specific changes in frequency receptive fields of single neurons in secondary and ventral ectosylvian auditory cortical fields. Brain Res 372:357–360
Divac I (1975) Magnocellular nuclei of the basal forebrain project to the neocortex brainstem and olfactory bulb. Review of some functional correlates. Brain Res 93:385–398
Donoghue JP, Carroll KL (1987) Cholinergic modulation of sensory responses in rat primary somatic sensory cortex. Brain Res 408:367–371
Edeline J-M, Weinberger NM (1991a) Subcortical adaptive filtering in the auditory system: associative receptive field plasticity in the dorsal medial geniculate body. Behav Neurosci 105:154–175
Edeline J-M, Weinberger NM (1991b) Thalamic short term plasticity in the auditory system: Associative retuning of receptive fields in the ventral medial geniculate body. Behav Neurosci 105(5): 618–639
Edeline J-M, Weinberger NM (1992) Associative retuning in the thalamic source of input to the amygdala and auditory cortex: receptive field plasticity in the medial division of the medial geniculate body. Behav Neurosci 106(1): 81–105
Fibiger HC (1982) The organization and some projections of cholinergic neurons of the mammalian forebrain. Brain Res 257:327–388
Fisher RS, Buchwald NA, Hull CD, Levine MS (1988) GABAergic basal forebrain neurons project to the neocortex: the localization of glutamic acid decarboxylase and choline acetyltransferase in feline corticopetal neurons. J Comp Neurol 272:489–502
Freund TF, Meskenaite V (1992) γ-Aminobutyric acid-containing basal forebrain neurons innervate inhibitory interneurons in the neocortex. Proc Natl Acad Sci USA 89:738–742
Hars B, Maho C, Edeline J-M, Hennevin E (1993) Basal forebrain stimulation facilitates tone-evoked responses in the auditory cortex of awake rat. Neuroscience 66:61–74
Hicks TP, Kaneko T, Metherate R, Oka JI, Stark CA (1991) Amino acids as transmitters of synaptic excitation in neocortical sensory processes. Can J Physiol Pharmacol 69:1099–1114
Imig TJ, Morel A (1985) Tonotopic organization in ventral nucleus of medial geniculate body in the cat. J Neurophysiol 53:309–340
Jacobs SE, Code RA, Juliano SL (1991) Basal forebrain lesions alter stimulus-evoked metabolic activity in rat somatosensory cortex. Brain Res 560:342–345
Jasper HH, Tessier J (1971) Acetylcholine liberation from cerebral cortex during paradoxical (REM) sleep. Science 172:601–602
Juliano SL, Ma W, Bear MF, Eslin D (1990) Cholinergic manipulation alters stimulus-evoked metabolic activity in cat somatosensory cortex. J Comp. Neurol 297(1): 106–20
Juliano SL, Ma W, Eslin D (1991) Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc Natl Acad Sci USA 88:780–784
Kelly JB, Sally SL (1988) Organization of the auditory cortex in the albino rat: binaural response properties. J Neurophysiol 59:1756–1769
Krnjevic K, Phillis JW (1963) Acetylcholine-sensitive cells in the cerebral cortex. J Physiol (Lond) 166:296–327
Kurosawa M, Sato A, Sato Y (1989) Stimulation of the nucleus basalis of Meynert increases acetylcholine release in the cerebral cortex in rats. Neurosci. Lett 98:45–50
Lamour Y, Dutar P, Jobert A (1982) Topographic organization of basal forebrain neurons projecting to the rat cerebral cortex. Neurosci Lett 34:117–122
Lamour Y, Dutar P, Jobert A, Dykes RW (1988) An iontophoretic study of single somatosensory neurons in rat granular cortex serving the limb: a laminar analysis of glutamate and acetylcholine effects on receptive field properties. J Neurophysiol 60:725–750
Langner G, Schreiner CE. (1988) Periodicity coding in the inferior colliculus of the cat. I. Neuronal mechanisms. J Neurophysiol 60(6): 1799–1822
Longo VG (1966) Effects of scopolamine and atropine on electroencephalographic and behavioral reactions due to hypothalamic stimulation. J Pharmacol 116:198–208
Luiten PGM, Gaykema RPA, Traber I, Spencer DGJ (1987) Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterograde transported phaseolus vulgaris leucoagglutinin. Brain Res 413:229–250
McCormick DA, Prince DA (1986) Mechanisms of action of acetylcholine in the guinea pig cerebral cortex in vitro. J Physiol (Lond) 375:169–194
McKenna TM, Ashe JH, Weinberger NM (1989) Cholinergic modulation of frequency receptive fields in auditory cortex: I. Frequency-specific effects of muscarinic agonists. Synapse 4(1): 30–43
Mesulam MM, Mufson EJ, Levey AI, Wainer BH (1983a) Cholinergic innervation of cortex by the basal forebrain: cytochemistry and cortical connexion of the septal area diagonal band nuclei, nucleus basalis (substantia innominata) and hypothalamus of the rhesus monkey. J Comp Neurol 214:170–197
Mesulam MM, Mufson EJ, Wainer BH, Levey AI (1983b) Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience 10(4): 1185–1201
Metherate R, Ashe JH (1991) Basal forebrain stimulation modifies auditory cortex responsiveness by an action at muscarinic receptors. Brain Res 559:163–167
Metherate R, Ashe JH (1993) Nucleus basalis stimulation facilitates thalamocortical synaptic transmission in rat auditory cortex. Synapse 14:132–143
Metherate R, Weinberger NM (1989) Acetylcholine produces stimulus-specific receptive field alterations in cat auditory cortex. Brain Res 480:372–377
Metherate R, Weinberger NM (1990) Cholinergic modulation of responses to single tones produces tone-specific receptive field alterations in cat auditory cortex. Synapse 6(2): 133–45
Metherate R, Tremblay N, Dykes R W (1987) Acetylcholine permits long-term enhancement of neuronal responsiveness in cat primary somatosensory cortex. Neuroscience 22(1): 75–81
Metherate R, Tremblay N, Dykes R W (1988a) The effects of acetylcholine on responses properties of cat somatosensory cortical neurons. J Neurophysiol 59:1231–1252
Metherate R, Tremblay N, Dykes R W (1988b) Transient and prolonged effects of acetylcholine on responsiveness of cat somatosensory cortical neurons. J Neurophysiol 59(4): 1253–1275
Metherate R, Cox CL, Acosta CG, Ashe JH (1989) Cholinergic agonists modify membrane potential and input resistance of auditory neocortical neurons in vitro (abstract). FASEB J 3:A393
Metherate R, Ashe JH, Weinberger NM (1990) Acetylcholine modifies neuronal acoustic rate-level functions in guinea pig auditory cortex by an action at muscarinic receptors. Synapse 6:364–368
Metherate R, Cox CL, Ashe JH (1992) Cellular bases of neocortical activation: modulation of neural oscillation by the nucleus basalis and endogenous acetylcholine. J Neurosci 12 (12) 4701–4711
Mitchell, JF (1963) The spontaneous and evoked release of acetylcholine. J Physiol (Lond) 165:98–116
Murphy PC, Sillito AM (1991) Cholinergic enhancement of direction selectivity in the visual cortex of the cat. Neuroscience 40:13–20
Paxinos G, Watson C (1982) The rat brain in stereotaxic coordinates. Academic, New York
Phillips DP, Orman SS, Musicant AD, Wilson GF (1985) Neurons in the cat's primary auditory cortex distinguished by their responses to tones and wide-spectrum noise. Hear Res 18:73–86
Phillis JR, Chong GC (1965) Acetylcholine release from the cerebral and cerebellar cortices: its role in cortical arousal. Nature 207:1253–1255
Popowitz JM, Larue DT, Winer JA (1988) Glutamate is major transmitter in the rat medial geniculate body. Soc Neurosci Abstr 14:490
Price JL, Stern R (1983) Individual cells in the nucleus basalis-diagonal band complex have restricted axonal projections to the cerebral cortex in the rat. Brain Res 269:352–356
Rasmusson DD, Dykes RW (1988) Long-term enhancement of evoked potentials in cat somatosensory cortex produced by coactivation of basal forebrain and cutaneous receptors. Exp. Brain Res 70:276–286
Redies H, Sieben U, Creutzfeldt OD (1989) Functional subdivisions in the auditory cortex of the guinea pig. J Comp Neurol 282:473–488
Riekkinen PJ, Riekkinen M, Sirviö J, Miettinen R, Riekkinen P (1992) Loss of cholinergic neurons in the nucleus basalis induces neocortical electroencephalic and passive avoidance deficits. Neuroscience 47:823–831
Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB (1984) Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 3:627–643
Saper CB (1984) Organization of cerebral cortical afferent systems in the rat. II Magnocellular basal nucleus. J Comp Neurol 222:313–342
Sato H, Hata Y, Hagihara K, Tsumoto T (1987a) Effects of cholinergic depletion on neurons activities in the cat visual cortex. J Neurophysiol 58:781–794
Sato M, Hata Y, Masui H, Tsumoto T (1987b) A functional role of cholinergic innervation to neurons in the cat visual cortex. J Neurophysiol 58:765–780
Sillito AM, Kemp JA (1983) Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res 289:143–155
Singer W (1979) Central-core control of visual cortex function. In: Schmitt FO, Worden FG (eds) The neurosciences fourth study program MIT, Cambridge, MA, pp 1093–1109
Steriade M, Buzsáki G (1990) Parallel activation of thalamic and cortical neurons by brainstem and basal forebrain cholinergic system. In: Steriade M, Biesold D (eds) Brain cholinergic systems. Oxford science, Oxford, pp 3–62
Steriade M, McCarley RW (1990) Brainstem control of wakefulness and sleep. Plenum, New York
Stewart DJ, Macfabe DF, Vanderwolf CH (1984) Cholinergic activation of the electrocorticogram: role of the substantia innominata and effects of atropine and quinuclidinyl benzilate. Brain Res 322:219–232
Szerb JC (1967) Cortical acetylcholine release and electroencephalographic arousal. J Physiol (Lond) 192:329–343
Tremblay N, Warren RA, Dykes RW (1990) Electrophysiological studies of acetylcholine and the role of the basal forebrain in the somatosensory cortex of the cat. II. Cortical neurons excited by somatic stimuli. J Neurophysiol 64(4): 1212–1222
Webster HH, Rasmusson DD, Dykes RW, Schliebs R, Schober W, Bruckner G, Bieslod D (1991) Long-term enhancement of evoked potentials in raccoon somatosensory cortex following co-activation of the nucleus basalis of Meynert complex and cutaneous receptors. Brain Res 545:292–296
Weinberger NM, Ashe JH, Metherate R, McKenna TM, Diamond DM, Bakin J (1990) Retuning auditory cortex by learning: a preliminary model of receptive field plasticity. Concepts Neurosci 1(1): 91–132
Wenk H, Bigl V, Meyer U (1980) Cholinergic projections from magnocellular nuclei of the basal forebrain to cortical areas in rats. Brain Res Rev 2:295–316
Woody CD, Swartz BE, Gruen E (1978) Effects of acetylcholine and cyclic GMP on input resistance of cortical neurons in awake cats. Brain Res 158:373–395
Woolf NJ (1991) Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol 37:475–524
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Edeline, J.M., Hars, B., Maho, C. et al. Transient and prolonged facilitation of tone-evoked responses induced by basal forebrain stimulations in the rat auditory cortex. Exp Brain Res 97, 373–386 (1994). https://doi.org/10.1007/BF00241531
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00241531