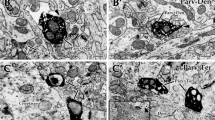
Abstract
This study investigates the synaptic relation between γ-aminobutyric acid-immunoreactive (GABA-IR) and cuneothalamic relay neurons (CTNs) in the rat cuneate nucleus. Retrograde transport of wheat germ agglutinin conjugated with horseradish peroxidase complex (WGA-HRP) was used to label CTNs while anti-GABA immunogold serum was used for the detection of GABA-IR boutons associated with CTNs. With these procedures, immunogold-labelled GABA-IR boutons were found to form axosomatic, axodendritic and axospinous synapses with the WGA-HRP-labelled but immunonegative CTNs. Quantitative estimation showed that the mean ratios of GABA-IR to GABA-immunonegative boutons making synaptic contacts with somata, proximal dendrites, and distal dendrites were 47.9%, 49.1% and 34.7%, respectively. Statistical analysis showed that the incidence of GABA-IR boutons on the somata and proximal dendrites of CTNs was significantly higher than on the distal dendrites. Our results indicate that GABA is the primary inhibitory neurotransmitter in the cuneate nucleus, thereby emphasizing the importance of postsynaptic inhibition on cuneothalamic relay neurons.
Similar content being viewed by others
References
Albe-Fessard D, Boivie J, Grant G, Levante A (1975) Labelling of cells in the medulla oblongata and the spinal cord of the monkey after injections of horseradish peroxidase in the thalamus. Neurosci Lett 1:75–80
Alger BE (1985) GABA and glycine: postsynaptic actions. In: Rogawski MA, Barker JL (eds) Amino acids. (Neurotransmitter actions in the vertebate nervous system, part I) Plenum, New York, pp 33–70
Andersen P, Eccles JC, Schmidt RF (1962) Presynaptic inhibition in the cuneate nucleus. Nature 194:741–743
Andersen P, Eccles JC, Oshima T, Schmidt RF (1964a) Mechanisms of synaptic transmission in the cuneate nucleus. J Neurophysiol 27:1096–1116
Andersen P, Eccles JC, Schmidt RF, Yokota T (1964b) Identification of relay cells and interneurons in the cuneate nucleus. J Neurophysiol 27:1080–1095
Andersen P, Etholm B, Gordon G (1970) Presynaptic and postsynaptic inhibition elicited in the cat's dorsal column nuclei by mechanical stimulation of skin. J Physiol (Lond) 210:433–455
Barbaresi P, Spreafico R, Frassoni C, Rustioni A (1986) GABAergic neurons ae present in the dorsal column nuclei but not in the ventroposterior complex of rats. Brain Res 382:305–326
Barker JL (1985) GABA and glycine: ion channel mechanisms. In: Rogawski MA, Barker JL (eds) Amino acids. (Neurotransmitter actions in the vertebrate nervous system, part I) Plenum, New York, pp 71–100
Basbaum AI, Hand PJ (1973) Projections of cervicothoracic dorsal roots to the cuneate nucleus of the rat, with observations on cellular “bricks”. J Comp Neurol 148:347–360
Berkley KJ, Budell RJ, Blomqvist A, Bull M (1986) Output systems of the dorsal column nuclei in the cat. Brain Res Rev 11:199–225
Biedenbach MA (1972) Cell density and regional distribution of cell types in the cuneate nucleus of the rhesus monkey. Brain Res 45:1–14
Boivie J (1978) Anatomical observations on the dorsal column nuclei, their thalamic projection and the cytoarchitecture of some somatosensory thalamic nuclei in the monkey. J Comp Neurol 178:17–48
Bowsher D (1958) Projection of the gracile and cuneate nuclei in Macaca mulatta: An experimental degeneration study. J Comp Neurol 110:135–155
Bromberg MB, Blum P, Whitehorn D (1975) Quantitative characteristics of inhibition in the cuneate nucleus of the cat. Exp Neurol 48:37–56
Davidoff RA, Hackman JC (1985) GABA: presynaptic actions. In: Rogawski MA, Barker JL (eds) Amino acids. (Neurotransmitter actions in the vertebrate nervous system, part I) Plenum, New York, pp 3–32
Davidson N, Smith CA (1972) A recurrent collateral pathway for presynaptic inhibition in the rat cuneate nucleus. Brain Res 44:63–71
Davidson N, Southwick CAP (1971) Amino acids and presynaptic inhibition in the rat cuneate nucleus. J Physiol (Lond) 219:689–708
Decavel C, VanDen Pol AN (1990) GABA: A dominant neurotransmitter in the hypothalamus. J Comp Neurol 302:1019–1037
Ellis LC Jr, Rustioni A (1981) A correlative HRP, Golgi, and EM study of the intrinsic organization of the feline dorsal column nuclei. J Comp Neurol 197:341–367
Galindo A (1969) GABA-picrotoxin interaction in the mammalian central nervous system. Bain Res 14:763–767
Galindo A, Krnjevic K, Schwartz S (1967) Micro-iontophoretic studies on neurones in the cuneate nucleus. J Physiol (Lond) 192:359–377
Graham RC Jr, Karnovsky MJ (1966) The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by new technique. J Histochem Cytochem 14:291–302
Hand PJ, Van Winkle T (1977) The efferent connections of the feline nucleus cuneatus. J Comp Neurol 171:83–110
Heino R, Westman J (1991) Quantitative analysis of the feline dorsal column nuclei and their GABAergic and non-GABAergic neurons. Anat Embryol 184:181–193
Horn AKE, Hoffmann KP (1987) Combined GABA-immunocytochemistry and TMB-HRP histochemistry of pretectal nuclei projecting to the inferior olive in rats, cats and monkeys. Brain Res 409:133–138
Isomura G, Hámori J (1988) Three types of neurons in the medial cuneate nucleus of the cat. Neurosci Res 5:395–408
Keller JH, Hand PJ (1970) Dorsal root projections to nucleus cuneatus of the cat. Brain Res 20:1–17
Kelly JS, Renaud LP (1973) On the pharmacology of ascending,descending and recurrent postsynaptic inhibition of the cuneothalamic relay cells in the cat. Br J Pharmacol 48:396–408
Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu JY, Ottersen OP, Storm-Mathisen J, Hama K (1987) Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res 66:191–210
Kuypers HGJM, Tuerk JD (1964) The distribution of the cortical fibres within the nuclei cuneatus and gracilis in the cat. J Anat 98:143–162
Levy RA, Anderson EG (1972) The effect of the GABA antagonists bicuculline and picrotoxin on primary afferent terminal excitability. Brain Res 43:171–180
Lue JH, Shieh JY, Chen KN, Wen CY (1993) Synaptic relationships between GABA-immunoreactive boutons and primary afferent terminals in the rat cuneate nucleus. Neuroscience 56:973–979
Pellegrino LJ, Pellegrino AS, Cushman AJ (1979) A stereotaxic atlas of the rat brain. Plenum, New York, pp 41–43
Roettger VR, Pearson JC, Goldfinger MD (1989) Identification of γ-aminobutyric acid-like immunoreactive neurons in the rat cuneate nucleus. Neurosci Lett 97:46–50
Rosén I (1969) Afferent connexions to group I activated cells in the main cuneate nucleus of the cat. J Physiol (Lond) 205:209–236
Rustioni A, Hayes NL, O'Neill S (1979) Dorsal column nuclei and ascending spinal afferents in macaques. Brain 102:95–125
Rustioni A, Schmechel DE, Cheema S, Fitzpatrick D (1984) Glutamic acid decarboxylase-containing neurons in the dorsal column nuclei of the cat. Somatosens Res 1:329–357
Rye DB, Saper CB, Wainer BH (1984) Stabilization of the tetramethylbenzidine (TMB) reaction product: application for retrograde and anterograde tracing, and combination with immunohistochemistry. J Histochem Cytochem 32:1145–1153
Shepherd GM, Koch C (1990) Introduction to synaptic circuits. In: Shepherd GM (ed) The synaptic organization of the brain. Oxford University Press, Oxford, pp 3–31
Towe AL, Jabbur SJ (1961) Cortical inhibition of neurons in dorsal column nuclei of cat. J Neurophysiol 24:488–498
Tan CK, Lieberman AR (1978) Identification of thalamic projection cells in the rat cuneate nucleus: a light and electron microscopic study using horseradish peroxidase. Neurosci Lett 10:19–22
Walberg F (1965) Axoaxonic contacts in the cuneate nucleus,probable basis for presynaptic depolarization. Exp Neurol 13:218–231
Walberg F (1966) The fine structure of the cuneate nucleus in normal cats and following interruption of afferent fibres. An electron microscopical study with particular reference to findings made in Glees and Nauta sections. Exp Brain Res 2:107–128
Wen CY (1984) The synaptic relationships between the primary afferent terminals and the cuneo-thalamic relay neurons in the rat cuneate nucleus. Proc Natl Sci Counc Repub China [B] 8:254–267
Wen CY, Chen KN, Lue JH, Chan SA, Shieh JY (1992) An electron microscopic and morphometric study on the GABA-immunoreactive terminals in the cuneate nucleus of the rat. J Anat 181:409–415
Wen CY, Wong WC, Tan CK (1978) The final structural organization of the cuneate nucleus in the monkey (Macaca fascicularis). J Anat 127:169–180
Westman J, Blomqvist A, Köhler C, Wu J-Y (1984) Light and electron microscopic localiztion of glutamic acid decarboxylase and substance P in the dorsal column nuclei of the cat. Neurosci Lett 51:347–352
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lue, J.H., Shieh, J.Y., Wen, C.Y. et al. GABAergic boutons establish synaptic contacts with the soma and dendrites of cuneothalamic relay neurons in the rat cuneate nucleus. Exp Brain Res 98, 13–20 (1994). https://doi.org/10.1007/BF00229104
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229104