Summary
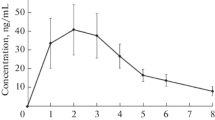
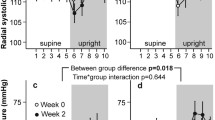
Prenalterol was studied in six healthy volunteers given single oral doses of 2.5, 5 and 10 mg and placebo. It displayed a distinct positive inotropic action, manifested as a dose-related reduction of 16.5–27.2 msec in the pre-ejection period (PEPc; systolic time-intervals), and an increase of 4.2–5.9 Ω/sec2 in the Heather index (impedance cardiography). There was also a dose-related increase of 17.6–34.0 mmHg in systolic blood pressure, whereas diastolic pressure showed a slight, transient decrease, not related to the dose given. Heart rate rose by 5–12 beats/min. Stroke volume, as determined by impedance cardiography, increased by 24.2–28.5 ml at all three dose-levels. The effects of the drug developed rapidly, reaching their maximum within 30–60 min and lasting for about 4 h. The time-course of the effects corresponded to the plasma concentrations of the drug. The increases in systolic pressure and contractility were linearly correlated with the plasma concentrations (r=0.8−0.9,p<0.001). The activity of prenalterol was also tested in the same volunteers after blockade of β-receptors with oxprenolol 80 mg. Under these conditions, oral doses of 25, 50 and 100 mg produced effects similar to or slightly less marked than those recorded after doses ten times lower in the absence of β-blockade. In a further 10 healthy volunteers, in whom tolerance to prenalterol was studied by repeated administration for 10 days of 5 mg four times daily, no change in blood chemistry, haematological parameters or urine values was found. The positive inotropic effect of a single oral dose of prenalterol 5 mg was also demonstrated by reference to the systolic time-intervals and the echocardiogram, in six patients with chronic heart failure, five of whom were digitalized. Prenalterol did not give rise to premature concentrations or other arrhythmias. The only untoward effect definitely attributable to the drug was palpitation, which was dose-related and as a rule was not unduly distressing; in one volunteer, however, the palpitations were unbearable. Prenalterol is a cardiostimulant agent with no direct effect on the peripheral circulation. On the basis of its pharmacological activity, it might well be of therapeutic benefit in all conditions in which an improvement in the pumping efficiency of the heart is required.
Similar content being viewed by others
References
Knaus M, Pfister B, Dubach UC, Imhof P (1978) Human pharmacology studies with a new, orally active stimulant of cardiac adrenergic beta-receptors. Am Heart J 95: 602–610
Johnsson G, Jordö L, Lundborg P, Rönn O, Welin-Fogelberg I, Wikstrand J (1978) Haemodynamic and tolerance studies in man of a new, orally active, selective β1-adrenoceptor agonist H 80/62. Eur J Clin Pharmacol 13: 163–170
Rönn O, Graffner C, Johnsson G, Jordö L, Lundborg P, Wikstrand J (1979) Haemodynamic effects and pharmacokinetics of a new selective beta1-adrenoceptor agonist, prenalterol, and its interaction with metoprolol in man. Eur J Clin Pharmacol 15: 9–13
Scott DHT, Arthur GR, Boyes RN, Scott DB (1979) Cardiovascular effects of prenalterol (H 133/22) in normal man. Br J Clin Pharmacol 7: 365–370
Ariniego R, Waagstein F, Mombay B, Hjalmarsson AC (1979) Haemodynamic effects of a new β1-receptor myocardial infarction. A useful antidote to β1-receptor blocking agents. Br Heart J 42: 139–146
Rönn O, Fellenius E, Graffner C, Johnsson G, Lundborg P, Svensson L (1980) Metabolic and haemodynamic effects and some pharmacokinetics of a new selective beta1-adrenoceptor agonist, prenalterol, in man. Eur J Clin Pharmacol 17: 81–86
Weissler AM, Harris WS, Schoenfeld CD (1969) Bed-side technics for the evaluation of ventricular function in man. Am J Cardiol 23: 577–583
Keller G, Imhof P (1975) The usefulness of impedance cardiography in human pharmacology studies. In: Assessment of pharmacodynamic effects in human pharmacology. Part II: Non-invasive methods in cardiovascular human pharmacology, Symposium Mainz, November 26, 1974. Schattauer, Stuttgart, pp 43–56
Weissler AM, Harris WS, Schoenfeld CD (1968) Systolic time intervals in heart failure in man. Circulation 37: 149–159
Kubicek WG, Karnegis JN, Patterson RP, Witsoe DA, Mattson RH (1966) Development and evaluation of an impedance cardiac output system. Aerospace Med 37: 1208–1213
Heather LW (1969) A comparison of cardiac output values by the impedance cardiograph and dye dilution technique in cardiac patients. Progress Report, NAS Contract No 9-4500, p 247
Degen PH, Errik M (1980) Determination of prenalterol in plasma and in urine by gasliquid chromatography. J Chromatogr submitted
Degen PH, Riess W (1976) Simplified method for the determination of oxprenolol and other β-receptor blocking agents in biological fluids by gas-liquid chromatography. J Chromatogr 121: 72–75
Holter NJ (1961) New method for heart studies. Science 134: 1214
Feigenbaum H (1976) Echocardiography, 2nd ed. Lea & Febiger, Philadelphia, pp 297–340
Brunner L, Imhof P, Jack D (1975) Relation between plasma concentrations and cardiovascular effects of oral oxprenolol in man. Eur J Clin Pharmacol 8: 3–9
CIBA-GEIGY Limited & Hässle AB (1977) Pharmacological report on H 133/22 — CGP 7760/B, a cardio-selective β-receptor stimulant.
Carlsson E, Ek L, Kopp U, Åblad B (1978) Prenalterol — A new orally active cardiac inotropic compound. VIIIth World Congress of Cardiology, Tokyo, September 17–23, Abstract No 1266, p 409
Mason DT (1978) Symposium perspective (Symposium on vasodilator and inotropic therapy of heart failure). Am J Med 65: 101–105
Parmley WW, Chatterjee K (1977) Combined vasodilator and inotropic therapy: A new approach in the treatment of heart failure. In: Mason DT (ed) Advances in heart disease, Vol I. Grune & Stratton, New York, pp 45–57
Cohn JN, Franciosa JA (1978) Selection of vasodilator, inotropic or combined therapy for the management of heart failure. Am J Med 65: 181–188
Jewitt D, Jennings K, Jackson PG (1978) Efficacy of new inotropic drugs in clinical coronary heart failure. Am J Med 65: 197–202
Imhof P, Garnier B (1965) Diagnose und Therapie der arteriellen Hypotension. Praxis 54: 1458–1465
Gotzen R (1978) Zur Therapie orthostatischer Kreislaufregulationsstörungen. Ther Ggw 117: 1214–1228
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weiss, A., Pfister, B., Imhof, P. et al. Haemodynamic effects, plasma concentrations and tolerance of orally administered prenalterol in man. Eur J Clin Pharmacol 18, 383–390 (1980). https://doi.org/10.1007/BF00636789
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00636789