Summary
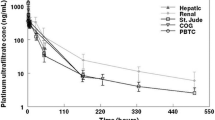
The pharmacokinetics of cyclophosphamide was investigated in 7 patients in severe liver failure. The pharmacokinetic data were compared with those derived from a matched control group of patients with normal liver function. The half-life (t1/2) of cyclophosphamide following intravenous administration in patients with liver failure was 12.5±1.0 h (m±SD), which was significantly longer than in the normal controls in whom it was 7.6±1.4 h (p<0.001). The mean total body clearance (Clt) was significantly smaller in liver failure at 44.8+8.6l·kg−1 than in the controls in whom it was 63.0±7.6l·kg−1 (p<0.01). It is concluded that severe liver disease has a significant effect on the disposition of cyclophosphamide, and that it could lead to accumulation of the drug in the body.
Similar content being viewed by others
References
Bagley CM, Bostic F, De Vita T (1973) Clinical Pharmacology of cyclophosphamide. Cancer Res 33: 226–233
Bosch NVD, Vos DD (1980) Some aspects of the gas-liquid chromatographic analysis of cyclophosphamide in plasma. J Chromatogr 183: 49–56
Colvin M (1978) A review of the pharmacology and clinical uses of cyclophosphamide. In: Pinedo (ed) Clinical pharmacology of antineoplastic drugs. Elsevier/North Holland, Biomedical Press, Amsterdam, pp 245–261
Fenselau C, Kan M-NN, Subba Rao, Myles A, Friedman OM, Colvin M (1977) Identification of aldophosphamide as a metabolite of cyclophosphamide in vitro and in vivo in humans. Cancer Res 37: 258–2543
Gibaldi M, Perrier D (1975) Pharmacokinetics. Marcel Dekker, New York
Jardine I, Fenselau C, Appler M, Kan MN, Brundrett RB, Colvin M (1978) Quantitation by gas chromatography-chemical ionisation mass spectrometry of cyclophosphamide, phosphoramide mustard and nor-nitrogen mustard in plasma and urine of patients receiving cyclophosphamide therapy. Cancer Res 38: 408–415
Jarman M, Milsted RAV, Smyth JF, Kinas RW, Pankiewicz K, Stec WT (1979) Comparative metabolism of 2-(bis (2-chloroethyl) amino) tetrahydro-2H-1, 3, 2-oxaza-phosphorine 2-oxide (cyclophosphamide) and entimers in humans. Cancer Res 39: 2762–2767
Juma FD, Rogers HJ, Trounce JR, Bradbrook ID (1978) Pharmacokinetics of intravenous cyclophosphamide in man, estimated by gas-liquid chromatography. Cancer Chemother Pharmacol 1: 229–231
Takamizawa A, Matsumoto S, Iwata T (1975) Studies on cyclophosphamide metabolites and their related compounds II. Preparation of an active species of cyclophosphamide and related compounds. J Med Chem 18: 376–383
Wagner T, Heydrich D, Bartels H, Hohorst HJ (1980) The influence of damaged liver parenchyma, renal insufficiency and haemodialysis on the pharmacokinetic of cyclophosphamide and its activated metabolites. Arzneimittelforsch 30: 1588–1592
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Juma, F.D. Effect of liver failure on the pharmacokinetics of cyclophosphamide. Eur J Clin Pharmacol 26, 591–593 (1984). https://doi.org/10.1007/BF00543491
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00543491