Summary
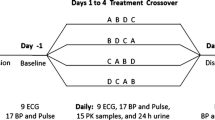
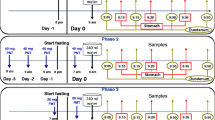
Enprofylline, a new potent bronchodilator xanthine drug, was given orally as an aqueous solution to 6 healthy subjects in single doses of 2, 4 and 6 mg/kg. The two lower doses produced plasma concentrations in the range 1–4 mg/l, i.e. in the assumed “therapeutic interval” according to previous animal studies. A high 24 h urine recovery of unchanged drug, with mean values for the three dose levels ranging from 85 to 91% of the given dose, indicated good absorption and little metabolism. The dose-corrected area under the plasma concentration-time curve rose with dose as the latter was increased from 2 to 6 mg/kg. This indicates that the elimination of enprofylline is capacity-limited at high doses. Double peaks in the plasma concentration-time curves at the higher dose levels suggested intermittent and delayed gastric emptying as a possible explanation. This hypothesis was confirmed by studies in 6 other healthy subjects, who received the drug solution by three different routes; by mouth, via a catheter in the duodenum, and rectally via a catheter in the colon. The corresponding time to peak values (mean±SEM) were 32.5±8.7, 13.3±2.5, and 157±23 min.
Similar content being viewed by others
References
Persson CGA, Kjellin G (1981) Enprofylline, a principally new antiasthmatic xanthine. Acta Pharmacol Toxicol 49: 313–316
Lunell E, Svedmyr N, Andersson K-E, Persson CGA (1982) Effects of enprofylline, a xanthine lacking adenosine receptor antagonism, in patients with chronic obstructive lung disease. Eur J Clin Pharmacol 22: 395–402
Lunell E, Andersson K-E, Persson CGA, Svedmyr N (1984) Intravenous enprofylline in asthma patients. Eur J Respir Dis 65: 28–34
Laursen LC, Johannesson N, Dirksen A, Djurup R, Munch EP, Taudorf E, Weeke B (1983) Enprofylline — effects of a new bronchodilating xanthine derivative in astmatic patients. Allergy 38: 75–79
Persson CGA, Karlsson J-A, Erjefält I (1982) Differentiation between bronchodilatation and universal adenosine antagonism among xanthine derivatives. Life Sci 30: 2181–2189
Lunell E, Svedmyr N, Andersson K-E, Persson CGA (1983) A novel bronchodilator xanthine apparently without adenosine receptor antagonism and tremorgenic effect. Eur J Respir Dis 64: 333–339
Persson CGA, Erjefält I, Edholm L-E, Karlsson J-A, Lamm CJ (1982) Tracheal relaxant and cardiostimulant actions of xanthines can be differentiated from diuretic and CNS-stimulant effects. Role of adenosine antagonism? Life Scie 31: 2673–2681
Borgå O, Andersson K-E, Edholm L-E, Fagerström P-O, Lunell E, Persson CGA (1983) Enprofylline kinetics in healthy subjects after single doses. Clin Pharmacol Ther 34: 799–804
Cutler DJ (1978) Theory of the mean absorption time, an adjunct to conventional bioavailability studies. J Pharm Pharmacol 30: 476–478
Laursen LC, Johannesson N, Fagerström P-O, Weeke B (1983) Intravenous administration of enprofylline to asthmatic patients. Eur J Clin Pharmacol 24: 323–327
Lunell E, Andersson K-E, Persson CGA (1983) Tolerance and some circulatory effects of intravenous and oral enprofylline in healthy volunteers. Acta Pharmacol Toxicol 53: 205–211
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lunell, E., Andersson, K.E., Borgå, O. et al. Absorption of enprofylline from the gastrointestinal tract in healthy subjects. Eur J Clin Pharmacol 27, 329–333 (1984). https://doi.org/10.1007/BF00542170
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00542170