Summary
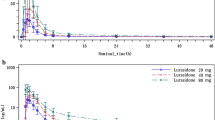
The pharmacokinetics of canrenone and ‘total metabolites’ after base hydrolysis was studied in eight young volunteers following single and multiple dose oral administration of spironolactone. The plasma levels of canrenone and ‘total metabolites’ were fitted to a two-compartment open model with a first-order absorption process. From our eight normal subjects studied, the harmonic mean of the distributive half-life (t1/2α) of canrenone was found to be 1.66 h, and the harmonic mean of the terminal elimination half-life (t1/2β) to be 22.6 h. Harmonic means of the distributive and elimination half-lives of ‘total metabolites’ after base hydrolysis were 2.48 h and 28.8 h respectively. The accumulation ratio of canrenone was 2.53, whereas that of ‘total metabolites’ was 1.89. Despite the fact that spironolactone has been shown to induce hepatic metabolism of other drugs, no evidence of autoinduction was noted in the present study, as plasma levels of canrenone and ‘total metabolites’ were found to obey a linear two-compartment model with reproducible absorption and disposition after single and multiple doses.
Similar content being viewed by others
References
Abshagen U, Besenfelder E, Endele R, Koch K, Neubert R (1979) Kinetic of canrenone after single and multiple doses of spironolactone. Eur J Clin Pharmacol 16: 255–262
Abshagen U, Rennekamp H, Luszpinski G (1976) Pharmacokinetics of spironolactone in man. Naunyn-Schmiedeberg's Arch Pharmacol 296: 37–45
Abshagen U, Rennekamp H, Luszpinski G (1977) Disposition kinetics of spironolactone in hepatic failure after single doses and prolonged treatment. Eur J Clin Pharmacol 11: 169–176
Colburn WA (1983) Estimating the accumulation of drugs. J Pharm Sci 72: 833–834
Dahlof CG, Lunborg P, Persson BA, Regardh CG (1979) Reevaluation of the antimineralocorticoid effect of the spironolactone metabolite, canrenone from plasma concentration determined by a new high pressure liquid chromatographic method. Drug Metab Dispos 7: 103–108
Gochman N, Gantt Cl (1962) A fluorimetric method for the determination of a major spironolactone (Aldactone) metabolite in human plasma. J Pharmacol Exp Ther 135: 312–316
Huston Gl, Turner P (1976) Antagonism of fludrocortisone by spironolactone and canrenone. Br J Clin Pharmacol 3: 201–204
Karim A, Ranney RE, Maibach HI (1971) Pharmacokinetic and metabolic fate of potassium canrenoate (SC-14266) in man. J Pharm Sci 60: 708–715
Karim A, Zagarella J, Hribar J, Dooley M (1976a) Spironolactone I. Disposition and metabolism. Clin Pharmacol Ther 19: 158–169
Karim A, Zagerella J, Hutsell TC, Chao A, Baltes BJ (1976b) Spironolactone II. Bioavailability. Clin Pharmacol Ther 19: 170–176
Karim A, Zagarella J, Hutsell TC, Dooley M (1976c) Spironolactone III. Canrenone — maximum and minimum steady-state plasma levels. Clin Pharmacol Ther 19: 177–181
Karim A (1978) Spironolactone: disposition, metabolism, pharmacodynamics and bioavailability. Drug Metab Rev 8: 151–188
McInnes GT, Asbury MJ, Ramsay LE, Shelton JR, Harrison IR (1982) Effect of micronization on the bioavailability and pharmacologic activity of spironolactone. J Clin Pharmacol 22: 410–417
Melander A, Danielsen K, Schersten B, Thulin T, Wahlin E (1977) Enhancement by food of canrenone bioavailability from spironolactone. Clin Pharmacol Ther 22: 100–103
Merkus FWHM, Overdiek JWPM, Cilissen J, Zuidema J (1983) Pharmacokinetics of spironolactone. After a single dose: evaluation of the true canrenone serum concentration during 24 hours. Clin Exper Hypertens A5(2): 239–248
Metzler CM, Elfring GL, McEwen AJ (1974) A package of computer programs for pharmacokinetic modeling. Biometrics 30: 562
Neubert P, Koch K (1977) Simultaneous automated determination of spironolactone metabolites in serum. J Pharm Sci 66: 1131–1134
Ramsay LE, Shelton JR, Wilkinson D, Tidd MJ (1976a) Canrenone — the principal active metabolite of spironolactone? Br J Clin Pharmacol 3: 607–612
Ramsay LE, Shelton J, Harrison I, Tidd M, Asbury M (1976b) Spironolactone and potassium canrenoate in normal man. Clin Pharmacol Ther 20: 167–177
Ramsay L, Asbury M, Shelton J, Harrison I (1977) Spironolactone and canrenoate-K: relative potency at steady state. Clin Pharmacol Ther 21: 602–609
Sadee W, Dagcioglu M, Riegelman S (1972) Fluorimetric microassay for spironolactone and its metabolites in biological fluids. J Pharm Sci 61: 1126–1129
Sadee W, Dagcioglu M, Schroder R (1973) Pharmacokinetics of spironolactone, canrenone and canrenoate-K in humans. J Pharmacol Exp Ther 185: 686–695
Sadee W, Schroder R, Leitner E, Dagcioglu M (1974) Multiple dose kinetics of spironolactone and canrenoate-potassium in cardiac and hepatic failure. Eur J Clin Pharmacol 7: 195–200
Sherry JH, O'Donnell JP, Colby HD (1981) Conversion of spironolactone to an active metabolite in target tissues: formation of 7-thiospironolactone by microsomal preparations from guinea pig liver, adrenals, kidneys, and testes. Life Sci 29: 2727–2736
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ho, P.C., Bourne, D.W.A., Triggs, E.J. et al. Pharmacokinetics of canrenone and metabolites after base hydrolysis following single and multiple dose oral administration of spironolactone. Eur J Clin Pharmacol 27, 441–446 (1984). https://doi.org/10.1007/BF00549592
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00549592