Summary
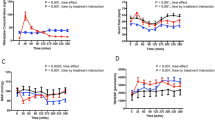
The efficacy of a transdermal (Nitroderm-TTS) and a transmucosal (Trinitrolong) nitroglycerin (NG) formulation has been compared with sublingual NG in 9 patients with ischaemic heart disease and stable angina pectoris. The duration and the degree of anti-ischaemic effect were assessed in terms of similar, individually adjusted work loads performed prior to and repeatedly after drug application in comparison with placebo. The anti-ischaemic effect of nitroderm appeared in 0.5–3 h after administration, reached a maximum in about 3.8 h and persisted for 7.9 h. The maximal nitroderm effect was significantly lower than that of sublingual NG or Trinitrolong. The effect of Trinitrolong was less variable and lasted for 4.6 h. It was evident in all patients 0.5 h after drug administration. Plasma NG levels were monitored in 9 patients after sublingual NG and trinitrolong and in 4 following Nitroderm. The relative bioavailability of Nitroderm and Trinitrolong according to the pharmacokinetic data was 29% and 256%, respectively, of sublingual NG tablets. A therapeutic NG level in blood (0.5 ng/ml) after Trinitrolong appeared much earlier (2 min) than after Nitroderm (1 h). A significant reduction in the effect of sublingual NG was observed during Nitroderm application. Thus, the transdermal NG formulation did not exhibit an antianginal effect lasting for 24 h; transmucosal NG had a relatively short, but more pronounced and stable antianginal effect.
Similar content being viewed by others
References
Breimer DD (1984) Rationale for rate-controlled drug delivery of cardiovascular drugs by transdermal route. Am Heart J 108: 196–200
Chein YW (1984) Pharmaceutical considerations of transdermal nitroglycerin delivery: the various approaches. Am Heart J 108: 207–216
Shaw JE (1984) Pharmacokinetics of nitroglycerin and clonidine delivered by the transdermal route. Am Heart J 108: 217–222
Cerri B, Grasso F, Cefis M, Pollavini G (1984) Comparative evaluation of the effect of two doses of Nitroderm TTS on exercise-related parameters in patients with angina pectoris. Eur Heart J 5: 710–715
Georgopoulos AJ, Markis A, Georgiadis H (1982) Therapeutic efficacy of a new transdermal system containing nitroglycerin in patients with angina pectoris. Eur J Clin Pharmacol 22: 481–485
Scardi S, Pivotti F, Fonda F, Pandullo C, Castelli M, Pollavini G (1985) Effect of a new transdermal therapeutic system containing nitroglycerin on exercise capacity in patients with angina pectoris. Am Heart J 110: 546–551
Crean PA, Ribeiro P, Crea F, Davies GJ, Ratcliffe D, Maseri A (1984) Failure of transdermal nitroglycerin to improve chronic stable angina: A randomized, placebo-controlled, doubleblind, double crossover trial. Am Heart J 108: 1494–1500
Parker JO, Fung H-L (1984) Transdermal nitroglycerin in angina pectoris. Am J Cardiol 54: 220–224
Reichek N, Priest C, Zimrin D, Chandler T, Sutton MSJ (1984) Antianginal effects of nitroglycerin patches. Am J Cardiol 54: 1–7
Abrams J (1983) New nitrate delivery systems: Buccal nitroglycerin. Am Heart J 105: 848–854
Metelitsa VI, Davydov AB, Savvateev KL, Khromov GL, Nikilenko SA, Martsevich SY (1980) Trinitrolong — a new preparation of prolonged action nitroglycerin. Ther Arch 5: 54–59
Redwood DR, Rosing DR, Goldstein RE, Beiser DG, Epstein SE (1971) Importance of the design of an exercise protocol in the evaluation of patients with angina pectoris. Circulation 43: 618–628
Metelitsa VI, Nikolenko SA, Nazarenko VA, Savvateev KL, Vygodin VA, Lupanov VP (1979) Pharmacodynamics of sustac and nitrong in patients with angina pectoris. Ther Arch 1: 40–49
Nazarenko VA, Nikolenko SA (1981) Method for studying the pharmacodynamics of anti-anginal drugs by means of repeated exercise tests. Kardiologya 1: 64–68
Blagodatskikh SV, Piotrovskii VK, Ryabokon OS, Martsevich SY, Metelitsa VI (1986) Comparative pharmacokinetics and bioavailability of isosorbide dinitrate from two formulations: Dinitrosorbilong and nitrosorbide. Khim-Farm Zh 1: 24–28
Naafs MA, de Boer AC, Koster RW, Klazen CW, Dunning AJ (1984) Exercise capacity with transdermal nitroglycerin in patients with stable angina pectoris. Eur Heart J 5: 705–709
Hollenberg M, Go M (1984) Clinical studies with transdermal nitroglycerin. Am Heart J 108: 223–231
Thadani U, Fung H-L, Darke A, Parker JO (1982) Oral isosorbide dinitrate in angina pectoris: Comparison of duration of action and dose-response relation during acute and sustained therapy. Am J Cardiol 49: 411–419
Bekhbudova DA, Kokurina EV, Ostrovskaya TP, Bochkareva EV, Urumbaev RK, Vygodin VA (1985) Characteristics of a group of patients subjected to prophylactic control in the district polyclinic and evaluation of antianginal therapy under dispensary conditions. Bull VKNTs 1: 95–100
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Metelitsa, V.I., Martsevich, S.Y., Piotrovskii, V.K. et al. New transdermal and transmucosal nitroglycerin delivery systems in patients with ischaemic heart disease. Eur J Clin Pharmacol 32, 5–10 (1987). https://doi.org/10.1007/BF00609950
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00609950