Summary
The pharmacokinetics and pharmacodynamics of a single oral dose benazepril·HCl 10 mg have been studied in 15 healthy volunteers aged 65 to 80 y. The kinetics of unchanged benazepril and its active metabolite benazeprilat did not differ significantly in males and females, so the combined kinetic data from all 15 elderly subjects were compared with a historical control group of 19–32 year-old healthy men treated in the same way.
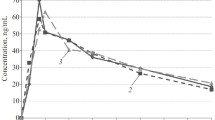
The disposition of benazepril was not affected by age. The time to maximum plasma concentration, tmax (0.5 h) and elimination half-life (0.6 h) in the elderly were the same as in young subjects. The kinetics of benazeprilat was slightly changed in the elderly; although its tmax (1.5 h) was not affected, Cmax and the AUC were 20–40% greater. The elimination half-life of benazeprilat during the first 24 h after doing in the elderly was increased by about 20% to 3.2 h. The renal plasma clearance of benazeprilat (18.1 ml·min−1) was about 20% smaller than in the young subjects. An average of 18.5% of the dose was recovered as benazeprilat in the 24 h urine from the elderly subjects, which was similar to the recovery in the young subjects. Both benazepril and benazeprilat were highly bound to serum proteins (96 and 95%, respectively).
Mean systolic and diastolic blood pressures in the elderly were reduced by a maximum of 37/16 mm Hg at 6 h, in association with a small rise in pulse rate.
Treatment was generally well tolerated. Three of the 15 subjects reported clinical adverse experiences judged to be possibly drug related, namely headache, abdominal pain and cold extremities.
Similar content being viewed by others
References
Kaiser G, Ackermann R, Sioufi A (1989) Pharmacokinetics of a new angiotensin-converting enzyme inhibitor, benazepril hydrochloride, in special populations. Am Heart J 117: 746–750
Hurley ME, Watters JT, Benz JR, Maher TF, Ivankoe LD, Ribeiro LGT (1987) CGS 14824 A, a new non-sulphhydryl angiotensin converting enzyme inhibitor (ACEI)-efficacy and safety in patients with mild to moderate hypertension. J Clin Pharmacol 27: 718
deSilva JK, Seshamani R, Ivankoe L, Chiang YT, Ribeiro LGT (1989) Benazepril as a safe and more effective alternative to diuretics in the treatment of mature hypertensive patients. Am J Hypertens 2 (5, part 2): 45A
Insel J, Mirvis DM, Boland MJ, Cinquegrani MP, Shanes J, Rubin SA, Whalen JJ (1989) A multicenter study of the safety and efficacy of benazepril hydrochloride, a long-acting angiotensin-converting enzyme inhibitor, in patients with chronic congestive heart failure. Clin Pharmacol Ther 45: 312–320
Waldmeier F, Schmid K (1989) Disposition of [14C]-benazepril hydrochloride in rat, dog and baboon: absorption, distribution, kinetics, biotransformation and excretion. Arzneim Forsch/Drug Res 39 (I): 62–67
Kaiser G, Ackermann R, Brechbühler S, Dieterle W (1989) Pharmacokinetics of the angiotensin converting enzyme inhibitor benazepril·HCl (CGS 14824 A) in healthy volunteers after single and repeated administration. Biopharm Drug Dispos 10: 365–376
Schaller MD, Nussberger J, Waeber B, Bussien JP, Turini GA, Brunner H, Brunner HR (1985) Haemodynamic and pharmacological effects of the converting enzyme inhibitor CGS 14824 A in normal volunteers. Eur J Clin Pharmacol 28: 267–272
Vestal RE (1978) Drug use in the elderly: A review of problems and special considerations. Drugs 16: 358–382
Temple R (1983) Memorandum to “Parties interested in clinical guidelines for the elderly” concerning “Discussion paper on testing of drugs in the elderly”. Department of Health and Human Services, Public Health Service, Food and Drug Administration, USA
Kaiser G, Ackermann R, Dieterle W, Dubois JP (1987) Determination of a new angiotensin converting enzyme inhibitor and its active metabolite in plasma and urine by gas chromatography-mass spectrometry. J Chromatogr 419: 123–133
Wagner J, Sulc M (1979) Bindung von Diclofenac-Na (Voltaren) an Serumproteine verschiedener Spezies und Interaktionen mit anderen Pharmaka. Akt Rheumatol 4: 153–162
Reid JL (1987) Angiotensin converting enzyme inhibitors in the elderly. Br Med J 295: 943–944
M'Buyamba-Kabangu JR, Fagard R, Lijnen P, Lijnen P, Staessen J, Van Hoof R, Amery A (1987) ACE-inhibitors in the treatment of elderly hypertensives. Geriatrics 42: 45–49
Creasey WA, Funke PT, McKinstry DN, Sugerman AA (1986) Pharmacokinetics of captopril in elderly healthy male volunteers. J Clin Pharmacol 26: 264–268
Cirillo VJ, Till AE, Gomez HJ, Ship WJ, Thieme G (1986) Effect of age on lisinopril pharmacokinetics. Clin Pharmacol Ther 39: 187
Gautam PC, Vargas E, Lye M (1987) Pharmacokinetics of lisinopril (MK 521) in healthy young and elderly subjects and in elderly patients with cardiac failure. J Pharm Pharmacol 39: 929–931
Hockings N, Ajayi AA, Reid JL (1986) Age and the pharmacokinetics of angiotensin converting enzyme inhibitors enalapril and enalaprilat. Br J Clin Pharmacol 21: 341–348
Lees KR, Reid JL (1987) Age and the pharmacokinetics and pharmacodynamics of chronic enalapril treatment. Clin Pharmacol Ther 41: 597–602
Ulm EH, Hichens M, Gomez HJ, Till AE, Hand E, Vassil TC, Biollaz J, Brunner HR, Schelling JL (1982) Enalapril maleate and a lysine analogue (MK-521): Disposition in man. Br J Clin Pharmacol 14: 357–362
Beerman B, Gomez H, Till A, Junggren IL (1986) Pharmacokinetics of lisinopril in healthy volunteers. Acta Pharmacol Toxicol 59 [Suppl V]: 66
Dieterle W, Ackermann R, Kaiser G (1989) Pharmacokinetics of benazeprilat after intravenous administration in healthy volunteers. Eur J Clin Pharmacol 36 [Suppl]: A 303
Author information
Authors and Affiliations
Additional information
Summary results were reported in the Proceedings of a Sattelite Symposium of the Xth Congress of the European Society of Cardiology, 1988, Vienna, Austria [1].
Rights and permissions
About this article
Cite this article
Kaiser, G., Ackermann, R., Dieterle, W. et al. Pharmacokinetics and pharmacodynamics of the ace inhibitor benazepril hydrochloride in the elderly. Eur J Clin Pharmacol 38, 379–385 (1990). https://doi.org/10.1007/BF00315579
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315579