Summary
Antacids can modify the pharmacokinetic parameters of sustained-release preparations of theophylline by changing the gastric pH. Though this has been studied with various theophylline/antacid combinations, the specific preparations investigated here have not previously been tested. The objective of the study was to assess any change in the availability of theophylline from a sustained-release preparation (SR), induced by the coadministration with an antacid.
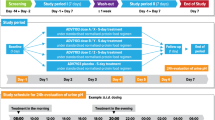
The study was designed as a double-blind randomized crossover trial in the Pneumology Departments of three general hospitals.
Fifteen patients were studied. They all had stable asthma treated with theophylline and no major organ failure or gastro-intestinal lesions requiring the use of antacids. The antacide (aluminium hydroxide 800 mg and magnesium hydroxide 800 mg), or placebo, tid, was added to a stable regimen of theophylline SR bid, for 4 days, in crossover fashion.
Plasma theophylline concentrations were measured before and 1,2,3,4,6,8,10,12,16 and 24 h after the morning dose of Armophylline on the fourth day of each treatment period; the maximum plasma concentration (Cmax), and time to Cmax (tmax) were noted, and the area under the 24-h time-concentration curve (AUC0–24) and mean plasma concentration (Cmean) were computed. Peak expiratory flows on the same day, before and 3, 6 and 12 h after the morning dose of Armophylline were also measured.
There was no change in any of the parameters studied.
The addition of the antacide to theophylline, each given according to standard clinical practice, did not modify the pharmacokinetics of the latter. This result probably can not be generalized to all pairs of sustained-release theophylline-antacid preparations.
Similar content being viewed by others
References
Jenne JW, Wyze E, Rood FS, MacDonald FM (1972) Pharmacokinetics of theophylline: application to the adjustment of the clinical dose of aminophyllin. Clin Pharmacol Ther 13: 349–360
Jacobs MH, Senior RM, Kessler G (1976) Clinical experience with theophylline: relationships between dosage, serum concentration and toxicity. JAMA 235: 1983–86
Rall T (1985) Central nervous system stimulants. In: Goodman A, Goodman L, Rall T, Murad F (eds) The pharmacological basis of therapeutics. Macmillan, New York, p 594
Nelson HS (1984) Gastroesophageal reflux and pulmonary disease. J Allergy Clin Immunol 73: 546–66
Hendeles L, Lafrate R, Weinberger M (1984) A clinical and pharmacokinetic basis for the selection and use of slow release theophylline products. Clin Pharmacokinet 9: 95–135
Hurwitz A (1977) Antacid therapy and drug kinetics. Clin Pharmacokinet 2: 269–280
Gugler R, Allgayer H (1990) Effects of antacids on the clinical pharmacokinetics of drugs: an update. Clin Pharmacokinet 18: 210–219
Staib A, Loew D, Harder S, Graul E, Pfab R (1986) Measurement of theophylline absorption from different regions of the gastrointestinal tract using a remote controlled drug delivery device. Eur J Clin Pharmacol 30: 691–697
D'Athis P (1990) Programme Triomphe®: traitement interactif ordonné des modèles pharmacologiques elémentaires. Département d'Informatique Médicale, CHU de Dijon, France
Darzentas L, Stewart R, Curry S, Yost R (1983) Effect of antacid on bioavailability of a sustained-release theophylline preparation. Drug Intell Clin Pharm 17: 555–557
Myhre K, Walstad R (1983) The influence of antacid on the absorption of two different sustained-release formulations of theophylline. Br J Clin Pharmacol 15: 683–687
Arnold L, Spurbeck G, Shelver W, Henderson W (1979) Effect of an antacid on gastrointestinal absorption of theophylline. Am J Hosp Pharm 36: 1059–1062
Reed R, Schwartz H (1984) Lack of influence of an intensive antacid regimen on theophylline bioavailability. J Pharmacokinet Biopharm 12: 315–331
Rohr W, Henderson W, Shelver W (1981) In vitro adsorption of theophylline by antacids. Am J Hosp Pharm 38: 1779–1780
De Sommers K, Meyer E, Van Wyk M, Moncrieff J (1980) Fraction of theophylline in sustained-release formulation which is absorbed from the large bowel. Eur J Clin Pharmacol 38: 171–173
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Muir, J.F., Peiffer, G., Richard, M.O. et al. Lack of effect of magnesium-aluminium hydroxide on the absorption of theophylline given as a pH-dependent sustained release preparation. Eur J Clin Pharmacol 44, 85–88 (1993). https://doi.org/10.1007/BF00315286
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00315286