Abstract
Methods: We investigated the pharmacokinetics of quinine (Qn) following administration of a single oral dose of 600 mg Qn sulphate in six male Thai patients with a moderate degree of chronic renal failure (CRF), and six male Thai subjects with normal renal function.
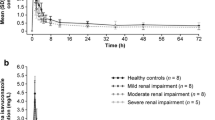
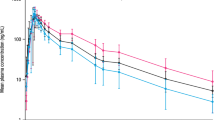
Results: The drug was well tolerated in both groups of subjects; no major adverse reactions were observed. A marked alteration in the pharmacokinetics of Qn was found in patients with CRF compared to healthy subjects; there were six signifiicant changes in the pharmacokinetic parameters. Absorption was delayed, but increased in CRF (tmax 4.5 vs 1.6 h, Cmax 6.17 vs 3.45 μg·ml−1). Total clearance was significantly reduced 0.94 vs 2.84 ml·min−1·kg−1, whereas Vz/f remained unchanged (1.82 vs 2.78 1·kg−1). This resulted in the increased values of AUC and prolongation of the t1/2z and MRT in the patients (AUC 181.5 vs 61.8 μg·min−1·ml−1, t1/2z 26 vs 9.7 h, MRT 36.4 vs 11.3 h). Median concentrations of plasma unbound fraction of Qn collected at 4 h after drug administration in patients and healthy subjects were 7.3 vs 9.8%, respectively.
Similar content being viewed by others
References
Bunnag D, Harinasuta T (1987) Quinine and quinidine in malaria in Thailand. Acta Leidensia 55:163–166
Warrell DA, Molyneux ME, Beales PF (eds) (1990) Severe and complicated malaria. Trans R Soc Trop Med Hyg 84 [Suppl]:1–65
Karbwang J, Harinasuta T (1992) Chemotherapy of malaria in Southeast Asia. Ruamtasana Co. Ltd., Bangkok
World Health Organization (1990) The clinical management of acute malaria. WHO Regional Publications, Southeast Asia Series No. 9
White NJ, Looareesuwan S, Warrell DA, Warrell MJ, Bunnag D, Harinasuta T (1982) Quinine pharmacokinetics and toxicity in cerebral and uncomplicated falciparum malaria. Am J Med 73:564–572
Karbwang J, Thanavibul A, Molunto P, Na-Bangchang K (1993) The pharmacokinetics of quinine in patients with hepatitis. Br J Clin Pharmacol 35:444–446
Auprayoon P, Sukontason K, Karbwang J, Banmairuroi V, Molunto P, Na-Bangchang K (1995) Pharmacokinetics of quinine in chronic liver disease. Br J Clin Pharmacol 40:494–497
Brodie BB, Baer JE, Craig LC (1951) Metabolic products of the cinchona alkaloids in human urine. J Biol Chem 88:567–581
Wattanagoon Y, Molunto P, Bunnag D, Na-Bangchang K, Karbwang J (1990) Effect of metoclopramide on quinine pharmacokinetics. Abstract “VIII International Congress of Parasitology. Paris, August 20–24, p 1073
Karbwang J, Na-Bangchang K, Molunto P, Bunnag D (1989) Determination of quinine and quinidine in biological fluids by high performance liquid chromatography. Southeast Asian J Trop Med Public Health 20:65–79
Silamut K, White NJ, Looareesuwan S, Warrell DA (1985) Binding of quinine to plasma proteins in falciparum malaria. Am J Trop Med Hyg 34:681–686
Gibaldi M (1991) Biopharmaceutics and clinical pharmacokinetics, 4th edn. Lee and Febiger, UK, pp 14–23
Notterman DA, Drayer DE, Metakis L, Reidenberg MM (1986) Stereoselective renal tubular secretion of quinidine and quinine. Clin Pharmacol Ther 4(5):511–517
Paap CM, Nahata MC (1993) The relation between type of renal disease and renal drug clearance in children. Eur J Clin Pharmacol 44:195–197
Trenholme GM, Williams RL, Rieckmann KH, Frisher H, Carson PE (1976) Quinine disposition during malaria and during induced fever. Clin Pharmacol Ther 19:459–467
Chau NP, Weiss YA, Safar ME, Lavene DE, Georges DR, Milliez PL (1977) Pindolol availability in hypertensive patients with normal and impaired renal function. Clin Pharmacol Ther 222:505–510
Tilstone WJ, Fine A (1978) Furosemide kinetics in renal failure. Clin Pharmacol Ther 23:499–508
Na-Bangchang K, Karbwang J, Bunnag D, Harinasuta T (1991) The effect of metoclopramide on mefloquine pharmacokinetics. Br J Clin Pharmacol 32:640–641
Reindenberg MM (1977) The biotransformation of drugs in renal failure. Am J Med 62:482–485
Verbeeck RK, Branch RA, Wilkinson GR (1981) Drug metabolites in renal failure. Clin Pharmacokinet 6:329–345
Krishna DR, Klotz U (1990) Basic pharmacokinetics elimination. In Krishna DR, Klotz U (eds) Clinical pharmacokinetics. Springer, Berlin Heidelberg New York, pp 38–57
Reindenberg MM, Drayer DE (1984) Alteration of drug protein binding in renal disease. Clin Pharmacokinet 9 [Suppl 1]:18–26
Haughey DB, Kraft CJ, Matzke GR, Keane WF, Halstenson CE (1985) Protein binding of disopyramide and elevated alpha-1-acid glycoprotein concentrations in serum obtained from dialysis patients and renal transplant recipients. Am J Nephron 5:35–39
Kim YG, Shin JG, Shin SG (1993) Decreased acetylation of isoniazid in chronic renal failure. Clin Pharmacol Ther 54:612–620
Mueller BA, Scarim SK, Macias WL (1993) Comparison of imipenam pharmacokinetics in patients with acute or chronic renal failure treated with continuous hemofiltration. Am J Kidney Dis 73:172–179
Guengerich FP, Muller-Enoch D, Blair IA (1986) Oxidation of quinidine by human liver cytochrome P-450. J Pharmacol Exp Ther 30:287–295
Anders MW (1980) Metabolism of drugs by the kidney. Kid Int 18:636–647
Gibson TP (1986) Renal disease and drug metabolism: an overview. Am J Kid Dis 8:7–17
Murray GI, Barnes TS, Sewell HF, Ewen SWB, Melvin WT, Burke MD (1988) The immunochemical localization and distribution of cytochrome P450 in normal human hepatic and extrahepatic tissues with a monoclonal antibody to human cytochrome P450. Br J Clin Pharmacol 25:165–175
Kolar JC, Schmiedin-Ren P, Scmiedin-Ren P, Schmiedin-Ren P, Schuetz JD, Watkins PB (1992) Identification of rifampicin-inducible p450IIIa4(cyp3a4) in human small bowel enterocytes. J Clin Invest 90:1871–1878
Dyson EH, Proudfoot AT, Prescott LF (1985) Death and blindness due to overdose of quinine. Br Med J 291:31–33
World Health Organization (1984) Advance in malaria chemotherapy. WHO Tech Rep Series 711:91–151
Hashiba K, Moss AJ, Schwartz PJ (1991) QT prolongation and ventricular arrhythmias. Ann New York Acad Sc 664 [Suppl]:1–247
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Rimchala, P., Karbwang, J., Sukontason, K. et al. Pharmacokinetics of quinine in patients with chronic renal failure. Eur J Clin Pharmacol 49, 497–501 (1996). https://doi.org/10.1007/BF00195937
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00195937