Abstract
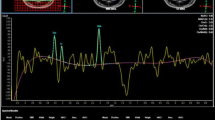
In 32 patients with gliomas, one- and two-dimensional proton magnetic resonance spectroscopy (1H-MRS) has been conducted, the latter allowing reconstruction of spectroscopic data into a spectroscopic image (MRSI), showing the distribution of the various metabolite concentrations over the cross-sectional plane. For lack of absolute concentrations, the measured concentrations of phosphocholine (CHOL),N-acetyl-L-aspartate (NAA), and lactate (LAC) were conventionally expressed in ratios relative to that of creatine (CREAT). Compared to normal brain tissue, an increased CHOL/CREAT ratio was found in all groups of tumours, in glioblastomas, high-, middle- and low-grade astrocytomas both at the margin and the core of the tumours, but in oligodendrogliomas only at the margin. This is consistent with an increased phosphocholine turnover in relation to membrane biosynthesis by the proliferating cells. The NAA/CREAT ratio was decreased in all groups of tumours, both in the centre and at the margin, reflecting replacement of functioning neurons by neoplastic cells. The LAC/ CREAT ratio was elevated in the core of malignant gliomas, which may be the result of a prevailing glycolysis, characteristic of tumours, possibly in conjunction with hypoxia/ischaemia. In the perifocal oedema, there was neither elevation of the CHOL/CREAT ratio nor decrease of the NAA/CREAT ratio; an increased LAC/CREAT ratio therefore rather reflected ischaemia/hypoxia probably due to locally elevated pressure and compromised regional perfusion. In the normal brain, the metabolite ratios of grey matter did not differ from those of white matter. The frontal lobe and basal ganglia showed lower NAA/CREAT ratios than the other cerebral areas. In 7 patients positron emission tomography was also performed with [18F]fluoro-2-deoxy-D-glucose (18FDG) or L-[1-11C]-tyrosine (11C-TYR); the latter demonstrated a pattern of11C-TYR uptake similar to that of CHOL elevation in the MRSI.
Similar content being viewed by others
References
Arnold DL, Matthews PM, Villemure JG (1989) Combined proton and phosphorus MRS of human brain tumours in vivo: preliminary observations on the effects of tissue heterogeneity on metabolite ratio and pH measurements (abstract). Proc Soc Magn Reson Med, 8th Annual Meeting, Berkeley, p 75
Ross BD, Merkle H, Staewen RS, Garwood M (1989) Spatially localized1H spectroscopy of a rat intracerebral glioma (abstract). Proc Soc Magn Reson Med, 8th Annual Meeting, Berkeley, p 146
Heerschap A, Luyten PR, Bernsen HJ, et al (1989) Combined1H and31P NMR spectroscopic examination of human intracranial tumours at 1.5 tesla (abstract). Proc Soc Magn Reson Med, 8th Annual Meeting, Berkeley, p 430
Sauter R, Loeffler W, Bruhn H, Frahm J (1989) High resolution localized proton MR spectroscopy at 1.0 Tesla (abstract). Proc Soc Magn Reson Med, 8th Annual Meeting, Berkeley, p 443
Felber S, Sauter R, Aichner F (1989) Localized 1H spectroscopy applied to cerebral neoplasms and ischaemia at a clinical 1.5 T MR-system (abstract). Proc Soc Magn Reson Med, 8th Annual Meeting, Berkeley, p 450
Gadian DG, Porteous R, Gill SS, et al (1989) Metabolites as1H NMR markers of brain disease (abstract). Proc Soc Magn Reson Med, 8th Annual Meeting, Berkeley, p 487
Bruhn H, Frahm J, Gyngell ML, et al (1989) Noninvasive differentiation of tumours with use of localized H-1 MR spectroscopy in vivo: initial experience in patients with cerebral tumours. Radiology 172: 541–548
Ott D, Ernst T, Hennig J (1990) Clinical value of1H spectroscopy of brain tumours (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 105
Remy C, Decorps M, Bourgeois D, Lefur Y, Devoulon P, Benabid AL (1989)1H spectroscopic imaging study of intracerebral tumour models in rat (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 136
Arnold DL, Villemure JG (1990) Clinical evaluation of primary brain tumours using in vivo magnetic resonance spectroscopy (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 990
Alger JR, Fulham JA, Frank JA, et al (1990) Metabolism of human glioma assessed by FGD-PET and1H-MRS in thirty patients (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 989
Luyten PR, Mariën AJH, Heindel W, Gerwen PHJ van, Herholz K, Hollander JA den, Friedmann G, Heiss WD (1990) Metabolic imaging of patients with intracranial tumours: H-1 MR spectroscopic imaging and PET. Radiology 176: 791–799
Kugel H, Heindel W, Ernestus RL, Bunke J, Friedmann G (1990) Imageguided localized1H NMR spectroscopy: a non-invasive tool for differentiation of human brain tumours? (abstract). Proc Soc Magn Reson Med, 10th Annual Meeting, Berkeley, p 589
Bizzi A, Fulham MJ, Sobering GS, et al (1991) Clinical evaluation of human gliomas with1H-MR spectroscopic imaging and [18F]-fluorodeoxyglucose PET (abstract). Proc Soc Magn Reson Med, 10th Annual Meeting, Berkeley, p 399
Frank JA, Alger JR, Bizzi A, et al (1990) Evaluation of low and high grade gliomas by proton MRS, gadolinium-DTPA enhanced MRI and FDG-PET: a study in 40 patients (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 102
Bottomley PA (1989) Human in vivo NMR spectroscopy in diagnostic medicine: clinical tool or research probe? Radiology 170: 1–15
Warburg O (1956) On the origin of cancer cells. Science 123: 309–314
Daemen BJG, Elsinga PH, Paans AMJ, Wieringa AR, Konings AWT, Vaalburg W (1992) Radiation-induced inhibition of tumour growth as monitored by PET using L-[1-11-C]-tyrosine and11FDG. J Nucl Med 33: 373–379
Hamacher K, Coenen HH, Stöcklin G (1986) Efficient stereospecific synthesis of no-carrier-added 2-[18F]fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med 27: 235–238
Bolster JM, Vaalburg W, Paans AMJ, et al (1986) Carbon-11C labelled tyrosine to study tumour metabolism by positron emission tomography (PET). Eur J Nucl Med 12: 321–324
Tunggal B, Hofmann K, Stoffel W (1990) In vivo13C nuclear magnetic resonance investigation of choline metabolism in rabbit brain. Magn Reson Med 13: 90–102
Hida K, Nakada T, Kwee IL (1990) Proton and31P spectroscopy of developing brain (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 1054
Hüppi PS, Posse S, Lazeyras F, et al (1991) Brain development in preterm and term babies studied with1H-magnetic resonance spectroscopy using short-echo time STEAM technique (abstract). Proc Soc Magn Reson Med, 10th Annual Meeting, Berkeley, p 377
Christiansen P, Larsson HBW, Frederiksen J, Jensen M, Henriksen O (1990) Localized in vivo proton spectroscopy in the brain of patients with multiple sclerosis (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 109
Rothman DL (1991) Brain spectroscopy of white matter diseases (abstract). Proc Soc Magn Reson Med, 10th Annual Meeting, Berkeley, p 78
Brenner RE, Williams SCR, Munro PMG, Barker GJ, Hawkins CP, McDonald WI (1991) The evolution of the in-vivo proton NMR spectrum in acute EAE (abstract). Proc Soc Magn Reson Med, 10th Annual Meeting, Berkeley, p 430
Larsson HBW, Christiansen P, Jensen M, et al (1991) Localized in vitro spectroscopy in the brain of patients with multiple sclerosis. Magn Res Med 22: 23–31
Bruhn H, Frahm J, Gyngell ML, Merboldt KD, Hänicke W, Sauter R (1989) Cerebral metabolism in man after acute stroke: new observations using localized proton NMR spectroscopy. Magn Reson Med 9: 126–131
Luyten PR, Rijen PC van, Tulleken CAF, Hollander JA den (1989) Metabolite mapping using1H NMR spectroscopic imaging in patients with cerebrovascular disease (abstract). Proc Soc Magn Reson Med, 8th Annual Meeting, Berkeley, p 452
Ott D, Hennig J (1990) In vivo1H spectroscopy in cerebral ischemia (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 1011
Duijn JH, Matson GB, Maudsley AA, Hugg JW, Weiner MW (1992) Human brain infarction: proton MR spectroscopy. Radiology 183: 711–718
Grodd W, Krägeloh-Mann I, Klose U, Sauter R (1990) Volume selective proton spectroscopy of the brain in children with neurodegenerative disorders (abstract). Proc Soc Magn Reson Med, 9th Annual Meeting, Berkeley, p 1050
Connelly A, Austin SJ, Gadian DG (1991) Localised1H MRS in the paediatric brain: age and regional dependence (abstract). Proc Soc Magn Reson Med, 10th Annual Meeting, Berkeley, p 379
Wang Z, Bogdan AR, Detre JA, Gusnard DA, Zimmerman RA (1991) In vivo1H MRS studies of pediatric metabolic disease (abstract). Proc Soc Magn Reson Med, 10th Annual Meeting, Berkeley, p 380
Barker PB, Kumar AJ, Naidu S (1991)1H NMR spectroscopy of Canavan's disease (abstract). Proc Soc Magn Reson Med, 10th Annual Meeting, Berkeley, p 381
Ishiwata K, Ido T, Nakajima T, Ohrui H, Kijima-Suda I, Itoh M (1990) Tumour uptake study of 18F-labeled N-acetylneuraminic acid. Int J Rad Appl Instrum [B] 17: 363–367
Ishiwata K, Tomura M, Ido T, Iwata R, Itoh J, Kameyama M (1989) In vivo assessment of 6-deoxy-6-[18F]fluoro-D-galactose as a PET tracer for studying galactose metabolism. Int J Rad Appl Instrum [B] 16: 775–781
Kugel H, Heindel W, Ernestus RI, Bunke J, Mesnil R du, Friedmann G (1992) Human brain tumours: spectral patterns detected with localized H-1 MR spectroscopy. Radiology 183: 701–709
Arnold DL, Shoubridge EA, Villemure JG, Feindel W (1990) Proton and phosphorus magnetic resonance spectroscopy of human astrocytomas in vivo: preliminary observations on tumour grading. NMR Biomed 3: 184–189
Oberhaensli RD, Hilton-Jones D, Bore PJ, Hands LJ, Rampling BP, Radda GK (1986) Biochemical investigation of human tumours in vivo with phosphorus-31 magnetic resonance spectroscopy. Lancet II: 8–11
Heindel W, Bunke J, Steinbrich W (1987) Image-guided localized31P NMR spectroscopy of the human brain at 1.5 T. Fortschr Röntgenstr 147: 374–378
Nadler JV, Cooper JR (1972) Metabolism of the aspartyl moiety ofN-acetyl-L-aspartic acid in the rat brain. J Neurochem 19: 2091–2105
D'Adamo AF, Yatsu FM (1966) Acetate metabolism in the nervous system.N-acetyl-L-aspartic acid and the biosynthesis of brain lipids. J Neurochem 13: 961–965
Birken DL, Oldendorf WH (1989)N-acetyl-L-aspartic acid: literature review of a compound prominent in1H-NMR spectroscopic studies of brain. Neurosci Biobehav Rev 13: 23–31
Go KG (1991) Cerebral pathophysiology. An integral approach with some emphasis on clinical implications. Elsevier, Amsterdam, pp 321–328
Patronas NJ, Di Chiro G, Kufta C et al (1985) Prediction of survival in glioma patients by means of positron emission tomography. J Neurosurg 62: 816–822
Kornblith PL, Cummins CJ, Smith BH, Brooks RA, Patronas NJ, Di Chiro G (1984) Correlation of experimental and clinical studies of metabolism by PET scanning. Prog Exp Tumour Res 27: 170–178
Graham JF, Cummins CJ, Smith BH, Kornblith PL (1985) Regulation of hexokinase in cultured gliomas. Neurosurgery 17: 537–542
Alger JR, Frank JA, Bizzi A, et al (1990) Metabolism of human gliomas: assessment with H-1 MR spectroscopy and F-18 fluorodeoxyglucose PET. Radiology 177: 633–641
Brooks JD, Beaney RP, Thomas DGT, Marshall J, Jones T (1986) Studies in regional cerebral pH in patients with cerebral tumours using continuous inhalation of11CO2 and positron emission tomography. J Cerebr Blood Flow Metab 6: 529–535
Buxton RB, Wechsler LR, Alpert NM, Ackerman RH, Elmaleh DR, Correia JA (1984) Measurement of brain pH using11CO2 and positron emission tomography. J Cerebr Blood Flow Metab 4: 8–16
Hochachka PW, Mommsen TP (1983) Protons and anaerobiosis. Science 219: 1391–1397
Heesters MAAM, Kamman RL, Mooyaart EL, Go KG (1993) Localized proton spectroscopy of inoperable brain gliomas. Response to radiation therapy. J Neuro-Oncol 17: 27–35
Ott D, Hennig J, Ernst T (1993) Human brain tumours: assessment with in vivo proton MR spectroscopy. Radiology 186: 745–752
Kayama T, Yoshimoto T, Fujimoto S, Sakurai Y (1991) Intratumoural oxygen pressure in malignant brain tumour. J Neurosurg 74: 55–59
Cruickshank GS, Rampling R (1994) Peritumoural hypoxia in human brain: peroperative measurement of the tissue oxygen tension around malignant brain tumours. Acta Neurochir [Suppl] 60: 375–380
Maier-Hauff K, Gerlach L, Cordes M (1990) HM-PAO-SPECT in the differentiation of cerebral gliomas. First experiences with blood flow measurements. In: Schneider GH, Vogler E, Kocever K (eds) Digitale Bildgebung, interventionelle Radiologie, integrierte digitale Radiologie. Blackwell, Oxford, pp 40–46
Go KG, Hew JW, Kamman RL, Molenaar WM, Pruim J, Blaauw EH (1993) Cystic lesions of the brain. A classification based on pathogenesis, with consideration of histological and radiological features. Eur J Radiol 17: 69–84
Van Vaals JJ, Bergman AH, Van den Boogert HJ, et al (1991) Non-invasive in vivo localised1H spectroscopy of human astrocytoma implanted in rat brain: regional differences followed in time. NMR Biomed 4: 125–132
Go KG, Keuter E, Kamman RL, Pruim J, Metzemaekers JDM, Staal MJ, Paans AMJ, Vaalburg W (1994) Contribution of magnetic resonance spectroscopic imaging and L-[11C]-tyrosine positron emission tomography to localization of cerebral gliomas for biopsy. Neurosurgery 34: 994–1002
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Go, K.G., Kamman, R.L., Mooyaart, E.L. et al. Localised proton spectroscopy and spectroscopic imaging in cerebral gliomas, with comparison to positron emission tomography. Neuroradiology 37, 198–206 (1995). https://doi.org/10.1007/BF01578258
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01578258