Abstract
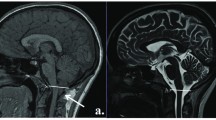
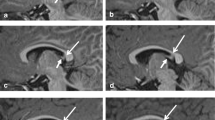
Our purpose was to establish parameters for normal infratentorial brain maturation at 0.5 and 1.5T and to evaluate the field strength criteria for the assessment of infratentorial brain maturation with MRI. We examined 27 children with normal psychomotor development (3 days to 24 months) with a 1.5T system and 22 (4 days to 29 months) with a 0.5T system; standard T2-weighted spin-echo sequences (TR/TE 2500/90 ms at 1.5T and TR/TE 2200/90 ms at 0.5T) were obtained. The signal intensity of infratentorial anatomical structures compared to their surroundings was classified as high, isointense or low by three neuroradiologists. For anatomical structures with age-related contrast changes, the time of these changes was determined statistically for the 0.5T and 1.5T system independently. The delineation of the structures without age-related contrast changes at the two field strengths was compared using a χ2 test. Age-related contrast changed were found in the same anatomical structures (“marker sites”) at 0.5 and 1.5T. Generally, these changes were apparent in larger structures (pons, middle cerebellar peduncles, medulla, cerebellar folia, red nuclei, cerebral peduncles), with only slight field-strength-dependent differences in the time of the contrast changes. Contrast changes from high to isointense signal were observed slightly earlier at 0.5T and changes from isointense to low signal slightly later at 0.5T. The delineation of the smaller anatomical structures was significantly better at 1.5T but these structures did not show age-related contrast changes. The differences in the assessment of infratentorial brain maturation between 0.5 and 1.5T can be attributed to a lower signal-to-noise ratio at lower magnetic field strengths. These differences do not complicate temporal classification of the stage of infratentorial brain maturation using the same “marker sites” and the same temporal criteria at 0.5 or 1.5T. However, higher field strengths are preferable for the assessment of smaller structures with physiological signal differences; this may imply better detection of small lesions at higher field strengths.
Similar content being viewed by others
References
Barkovich AJ, Kjos BO, Jackson DE, et al (1988) Normal maturation of the neonatal infant brain: MR imaging at 1.5T. Radiology 166:173–180
Knapp MS van der, Valk J (1990) MR imaging of various stages of normal myelination during the first year of life. Neuroradiology 31:459–470
Bird CR, Hedberg M, Drayer BP, Keller PJ, Flom RA, Hodak JA (1989) MR assessment of myelination in infants and children: usefulness of marker sites. AJNR 10:731–740
Martin E, Krassnitzer S, Kaelin P, Boesch C (1991) MR imaging of the brainstem: normal postnatal development. Neuroradiology 33:391–395
Stricker T, Martin E, Boesch C (1990) Development of the human cerebellum observed with high-field-strength MR imaging. Radiology 177:431–435
Sarnat HB (1992) Developmental disorders of the posterior fossa structures. In: Sarnat HB (ed) Cerebral dysgenesis — embryology and clinical expression. Oxford University Press, Oxford, pp 316–318
Lotspeich LJ, Ciaranello RD (1993) The neurobiology and genetics of infantile autism. Int Rev Neurobiol 35: 87–129
Nunez J, Couchie D, Aniello F, Brioux AM (1992) Thyroid hormone effects on neuronal differentiation during brain development. Acta Med Austriaca 19 [Suppl 1]: 36–39
Ward GR, Wainwright PE (1991) Effects of prenatal stress and ethanol on cerebellar fiber tract maturation in B6D2F2 mice: an image study. Neurotoxicology 12:665–676
Chen CN, Sank VJ, Cohen SM, Hoult DI (1986) The field dependence of NMR imaging. I. Laboratory assessment of signal-to-noise ratio and power depostition. Magn Reson Med 3:722–729
Hoult DI, Chen CN, Sank VJ (1986) The field dependence of NMR imaging. II. Arguments concerning an optimal field strength. Magn Reson Med 3:730–746
Barkovich AJ, Truwit CL (1990) Practical MRI atlas of neonatal brain development. Raven Press, New York, pp 3–52
Hittmair K, Wimberger D, Bernert G, Rand T, Kramer J, Imhof H (1994) MR assessment of brain maturation: comparison of SE and STIR sequences. AJNR 15:425–433
Maezawa M, Seki T, Imura S, Akiyama K, Takikawa I, Yuasa Y (1993) Magnetic resonance signal intensity ratio of gray/white matter in children (quantitative assessment in developing brain). Brain Dev 15:198–205
Hirsch WL, Kemp SS, Martinez AJ, Curtin H, Latchaw RE, Wolf G (1989) Anatomy of the brainstem: correlation of in vitro MR images with histologic sections. AJNR 10:923–928
Kretschmer HJ, Weinrich W (1992) Cranial neuroimaging and clinical neuroanatomy. Thieme, Stuttgart, pp 142–143
Martin E, Boesch C, Zuerrer M, et al (1990) MR imaging of brain maturation in normal and developmentally handicapped children. J Comput Assist Tomogr 14:685–692
Sachs L (1970) Statistische Methoden. Springer, Berlin Heidelberg New York, S 64–66
Schima W, Wimberger D, Schneider B, Stiglbauer R, Asenbaum S, Imhof H (1993) Field strength in MR imaging of multiple sclerosis: a comparison of 0.5 and 1.5T. ROFO 158:368–371
Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM (1984) A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms for 1–100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med Phys 11: 425–448
Aoki S, Okada Y, Nishimura K, Barkovich A, Kjos BO, Brasch RC, Norman D (1989) Normal deposition of brain iron in childhood and adolescence: MR imaging at 1.5T. Radiology 172:381–385
Jack CR Jr, Berquist TH, Miller GM, Forbes GS, Gray JE, Morin RL, Ilstrup DM (1990) Field strength in neuro-MR imaging: a comparison of 0.5T and 1.5T. J Comput Assist Tomogr 14:505–513
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hittmair, K., Kramer, J., Rand, T. et al. Infratentorial brain maturation: a comparison of MRI at 0.5 and 1.5T. Neuroradiology 38, 360–366 (1996). https://doi.org/10.1007/BF00596589
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00596589