Abstract
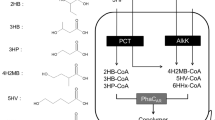
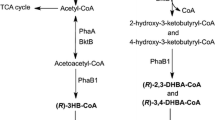
Pseudomonas sp. A33 and other isolates of aerobic bacteria accumulated a complex copolyester containing 3-hydroxybutyric acid (3HB) and various medium-chain-length 3-hydroxyalkanoic acids (3HAMCL) from 3-hydroxybutyric acid or from 1,3-butanediol under nitrogen-limitated culture conditions. 3HB contributed to 15.1 mol/100 mol of the constituents of the polyester depending on the strain and on the cultivation conditions. The accumulated polymer was a copolyester of 3HB and 3HAMCL rather than a blend of poly(3HB) and poly(3HAMCL) on the basis of multiple evidence. 3-Hydroxyhexadecenoic acid and 3-hydroxyhexadecanoic acid were detected as constituents of polyhydroxyalkanoates, which have hitherto not been described, by13C nuclear magnetic resonance or by gas chromatography/mass spectrometric analysis. In total, ten different constituents were detected in the polymer synthesized from 1,3-butanediol by Pseudomonas sp. A33:besides seven saturated (3HB, 3-hydroxyhexanoate, 3-hydroxyoctanoate, 3-hydroxydecanoate, and 3-hydrohexadecanoate) three unsaturated (3-hydroxydodecenoate, 3-hydroxytetradecenoate and 3-hydrohexadecanoate) hydroxyalkanoic acid constituents occured. The polyhydroxyalkanoate synthase of Pseudomonas sp. A33 was cloned, and its substrate specificity was evaluated by heterologous expression in various strains of P. putida, P. oleovorans and Alcaligenes eutrophus.
Similar content being viewed by others
References
Anderson AJ, Dawes EA (1990) Occurrence, metabolism, metabolic role and industrial uses of bacterial polyhydroxyalkanoates. Microbial Rev 54:450–472
Ballistreri A, Montaudo G, Impallomeni G, Lenz RW, Kim YB, Fuller RC (1990) Sequence distribution of β-hydroxyalkanoate units with higher alkyl groups in bacterial copolyesters. Macromolecules 23:5059–5064
Brandl H, Hamer GK, Lenz RW, Fuller RC (1988) Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegrable polyesters. Appl Environ Microbiol 54:1977–1982
Byrom D (1987) Polymer synthesis by microorganisms: technology and economics. Trends Biotechnol 5:246–250
Delafield FP, Doudoroff M, Palleroni NJ, Lusty CJ, Contopoulos R (1965) Decomposition of poly-β-hydroxybutyrate by pseudomonads. J Bacteriol 90:1455–1466
Deretic V, Chandrasekharappa JF, Gill JF, Chatterjee DK, Chakrabarty AM (1987) A set of cassettes and improved vectors for genetic and biochemical characterization of Pseudomonas genes. Gene 57:61–72
Doi Y, Kunioka M, Nakamura Y, Soga K (1986) Nuclear magnetic resonance studies on poly(β-hydroxybutyrate) and a copolyester of β-hydroxybutyrate and β-hydroxyvalerate isolated from Alcaligenes eutrophus H16. Macromolecules 19:2860–2864
Doi Y, Tamaki A, Kunioka M, Soga K (1987) Biosynthesis of ter-polyesters of 3-hydroxybutyrate, 3-hydroxyvalerate and 5-hydroxyvalerate in Alcaligenes eutrophus from 5-chloropentanoic and pentanoic acids. Makromol Chem Rapid Commun 8:631–635
Eggink G, Waard P de, Huijberts GNM (1992) The role of fatty acid biosynthesis and degradation in the supply of substrates for poly(3-hydroxyalkanoate) formation in Pseudomonas putida. FEMS Microbial Rev 103:159–164
Friedrich B, Hogrefe C, Schlegel HG (1981) Naturally occurring genetic transfer of hydrogen-oxidizing ability between strains of Alcaligenes eutrophus. J Bacteriol 147:198–205
Fritzsche K, Lenz RW, Fuller RC (1990a) An unusal bacterial polyester with a phenyl pendant group. Makromol Chem 191:1957–1965
Fritzsche K, Lenz RW, Fuller RC (1990b) Bacterial polyesters containing branched poly(β-hydroxyalkanoate) units. Int J Biol Macromol 12:92–101
Fritzsche K, Lenz RW, Fuller RC (1990c) Production of unsaturated polyesters by Pseudomonas oleovorans. Int J Biol Macromol 12:85–91
Gross RA, Demello C, Lenz RW, Brandl H, Fuller RC (1989) Biosynthesis and characterization of poly(β-hydroxyalkanoates) produced by Pseudomonas oleovorans. Macromolecules 22:1106–1115
Haywood GW, Anderson AJ, Dawes EA (1989) The importance of PHB-synthase substrate specificity in polyhydroxyalkanoate synthesis by Alcaligenes eutrophus. FEMS Microbiol Lett 57:1–6
Haywood GW, Anderson AJ, Ewing DF, Dawes EA (1990) Accumulation of a polyhydroxyalkanoate containing primarily 3-hydroxydecanoate from simple carbohydrate substrates by Pseudomonas sp. strain NCIMB40135. Appl Environ Microbiol 56:3354–3359
Hohn B, Collins J (1980) A small cosmid for efficient cloning of large DNA fragments. Gene 11:291–298
Holmes PA (1985) Applications of PHB—a microbially produced biodegradable thermoplastic. Phys Technol 16:32–36
Huijberts GNM, Eggink G, Waard P de, Huisman GW, Witholt B (1992) Pseudomonas putida KT2442 cultivated on glucose accumulates poly(3-hydroxyalkanoates) consisting of saturated and unsaturated monomers. Appl Environ Microbiol 58:1949–1954
Huisman GW, Leeuw O de, Eggink G, Witholt B (1989) Synthesis of poly-3-hydroxyalkanoates is a common feature of fluorescent pseudomonads. Appl Environ Microbiol 55:1949–1954
Huisman GW, Wonik E, Meinma R, Kazemier B, Terstra P, Witholt B (1991) Metabolism of poly(3-hydroxyalkanotes) by Pseudomonas oleovorans: identification and sequence of genes and function of the encoded proteins in the synthesis and degradation of PHA. J Biol Chem 266:2191–2198
Jendrossek D, Knoke I, Habibian RB, Steinbüchel A, Schlegel HG. (1993) Degradation of poly(3-hydroxybutyrate), PHB, by bacteria and purification of a novel PHB depolymerase from Comamonas sp. J Environ Polym Degrad 1:53–63
Kunioka M, Nakamura Y, Doi Y (1988) New bacterial copolyesters produced in Alcaligenes eutrophus from organic acids. Polym Commun 29:174–176
Lageveen RG, Huisman GW, Preusting H, Ketelaar P, Eggink G, Witholt B (1988) Formation of polyesters by Pseudomonas oleovorans: effect of substrates on formation and composition of poly-(R)-3-hydroxyalkenoates. Appl Environ Microbiol 54:2924–2932
Lemoigne, M. (1926) Produits de deshydration et de polymerisation de l'acide β-oxybutyrique. Bull Soc Chim Biol 8:770–782
Lenz RW, Kim BW, Ulmer HW, Fritzche K (1990) Functionalized poly-β-hydroxyalkanoates produced by bacteria. In: Dawes EA (ed) Novel biodegradable microbial polymers. Kluwer, Dordrecht, pp 23–35
Liebergessell M, Mayer F, Steinbüchel A (1993) Analysis of polyhydroxyalkanoic acid-biosynthesis genes of anoxygenic phototrophic bacteria reveals synthesis of a polyester exhibiting an unusual composition. Appl Microbiol Biotechnol 40:292–300
McLafferty FW (1966) Interpretation of mass spectra, 1st edn. Benjamin, New York
Müller HM, Seebach D (1993) Poly(hydroxyfettsäureester), eine fünfte Klasse von physiologisch bedeutsamen organischen Biopolymeren? Angewandte Chem 105:483–658
Nakamura S, Kunioka M, Doi Y (1991) Biosynthesis and characterization of bacterial poly(3-hydroxybutyrate-co-3-hydroxypropionate). Macromol Rep A28:15–24
Preusting H, Kingma J, Huisman GW, Steinbüchel A, Witholt B (1992) Formation of polyester blends by a recombinant strain of Pseudomonas oleovorans: different poly(3-hydroxyalkanoates) are stored in separate granules. J Environ Polym Degrad 1:11–21
Preusting H, Nijenhuis A, Witholt B (1990) Physical characterizations of poly(3-hydroxyalkanoates) produced by Pseudomonas oleovorans grown on aliphatic hydrocarbons. Macromolecules 23:4220–4224
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory PressCold Spring Harbor, New York
Schirmer A, Jendrossek D, Schlegel HG (1993) Degradation of poly(3-hydroxyoctanoic acid) [P(3HO)] by bacteria: purification and properties of a P(3HO) depolymerase from Pseudomonas flurescens GK13. Appl Environ Microbiol 59:1220–1227
Schlegel HG, Kaltwasser H, Gottschalk G (1961) Ein Submersverfahren zur Kultur wassertoffoxidierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch Mikrobiol 38:209–222
Schlegel HG, Lafferty RM, Krauss J (1970) The isolation of mutants not accumulating poly-β-hydroxybutyric acid. Arch Mikrobiol 71:283–294
Simon R, Priefer U, Pühler A (1983) A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology 1:784–791
Steinbüchel A (1991a) Polyhydroxyalkanoic acids. In: D Byrom (ed) Biomaterials. Macmillan, New York, pp 123–213
Steinbüchel A (1991b) Recent advances in the knowledge of bacterial poly(hydroxyalkanoic acid) metabolism and potential impacts on the production of biodegradable thermoplastics. Acta Biotechnol 11:419–427
Steinbüchel A, Schlegel HG (1991) Physiology and molecular genetics of poly(β-hydroxyalkanoic acid) synthesis in Alcaligenes eutrophus. Mol Microbial 5:535–542
Steinbüchel A, Wiese S (1992) A Pseudomonas strain accumulating polyesters of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids. Appl Microbiol Biotechnol 37:691–697
Steinbüchel A, Hustede E, Liebergesell M, Pieper U, Timm A, Valentin H (1992) Molecular basis for biosynthesis and accumulation of polyhydroxyalkanoic acids in bacteria. FEMS Microbiol Rev 103:217–230
Timm A, Steinbüchel A (1990) Formation of polyesters consisting of medium-chain-length 3-hydroxyalkanoic acids from gluconate by Pseudomonas aeruginosa and other fluorescent Pseudomonads. Appl Environ Microbiol 56:3360–3367
Timm A, Steinbüchel A (1992) Cloning and molecular analysis of the poly(3-hydroxyalkanoic acid) gene locus of Pseudomonas aeruginosa PAO1. Eur J Biochem 209:15–30
Timm A, Byrom D, Steinbüchel A (1990) Formation of blends of various poly(3-hydroxyalkanoic acids) by a recombinant strain of Pseudomonas oleovorans. Appl Microbiol Biotechnol 33:296–301
Timm A, Wiese S, Steinbüchel A (1994) A general method for identification of polyhydroxyalkanoic acid synthase genes from pseudomonads belonging to the rRNA homology group I. Appl Microbiol Biotechnol 40:669–675
Valentin HE, Steinbüchel A (1994) Application of enzymatically synthesized short-chain-length hydroxy fatty acid coenzyme A thioesters for assay of polyhydroxyalkanoic acid synthases. Appl Microbiol Biotechnol 40:699–709
Valentin HE, Lee EY, Choi CY, Steinbüchel A (1994) Identification of 4-hydroxyhexanoic acid as a new constituent of biosynthetic polyhydroxyalkanoic acids from bacteria. Appl Microbiol Biotechnol 40:710–716
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lee, E.Y., Jendrossek, D., Schirmer, A. et al. Biosynthesis of copolyesters consisting of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids from 1,3-butanediol or from 3-hydroxybutyrate by Pseudomonas sp. A33. Appl Microbiol Biotechnol 42, 901–909 (1995). https://doi.org/10.1007/BF00191189
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00191189