Abstract
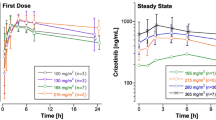
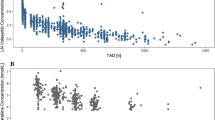
Purpose: We studied vincristine disposition after 169 weekly i.v. bolus injections in 32 children with acute lymphoblastic leukemia, non-Hodgkin lymphoma, or Wilms' tumor. The aim of the study was to determine intrapatient and interpatient variability in vincristine disposition and demographic, clinical, and biochemical characteristics influencing this variability. Methods: Vincristine plasma concentrations were measured by a high-performance liquid chromatography assay with electrochemical detection. A limited sampling strategy was used based on a bayesian parameter estimation algorithm that is part of the ADAPT II software package. A two-compartment, first-order model was fitted to the data, and pharmacokinetic parameters were calculated from the model using the ADAPT II software. For statistical analysis, analysis of variance (ANOVA), t test, simple and multiple regression analysis, and non-parametric or robust equivalents were used. Results: Results showed a large intrapatient and interpatient variability in distribution half-life, elimination half-life, total body clearance, apparent volume of distribution at steady state, and area under the concentration–time curve. Intrapatient variability was significantly smaller than interpatient variability for all these parameters except distribution half-life. The diagnosis or treatment protocol turned out to be the most predictive characteristic; leukemia and non-Hodgkin lymphoma patients had a significantly higher total body clearance than Wilms' tumor patients. Conclusions: We conclude that both intrapatient and interpatient variability in vincristine pharmacokinetics is large in pediatric cancer patients and that variability, although significantly influenced by diagnosis, largely remains unpredictable.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 11 September 1998 / Accepted: 5 February 1999
Rights and permissions
About this article
Cite this article
Gidding, C., Meeuwsen-de Boer, G., Koopmans, P. et al. Vincristine pharmacokinetics after repetitive dosing in children. Cancer Chemother Pharmacol 44, 203–209 (1999). https://doi.org/10.1007/s002800050968
Issue Date:
DOI: https://doi.org/10.1007/s002800050968