Summary
The pharmacokinetic and bioavailability properties of medroxyprogesterone acetate (MPA) after single PO and IM doses in man were used as a basis to predict, on a theoretical pharmacokinetic basis, the blood level profile of the drug during repeated dose administration with various dosage schedules.
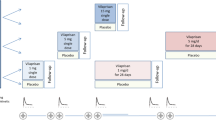
Because of the unusually long-lasting depot effect of IM MPA, a different build-up process of blood levels is expected during repeated IM or PO administration, and this should be taken into account when dose schedules for use in clinical oncology are selected. As regards the IM route, dose schedules based on 4 weeks' treatment with daily injections of 500–1,000 mg followed by a maintenance therapy with 1,000 mg/week are suggested, since they permit rapid achievement and maintenance of relatively high plasma levels.
A similar plasma level profile can be obtained with oral MPA provided that daily doses twice as large as the IM doses are given during the first month of treatment and continued during the maintenance period. The serum levels observed in 25 patients with advanced breast cancer treated with MPA given IM or PO according to various dose schedules and recent literature data are very close to the serum level profiles predicted on a theoretical pharmacokinetic basis.
Similar content being viewed by others
References
Bonte J, Decoster JM, Ide P, Billiet G (1978) Hormonoprophylaxis and hormonotherapy in the treatment of endometrial carcinoma by means of medroxyprogesterone acetate. Gynecol Oncol 6:60–75
Cavalli F, Alberto P, Martz G, Varini M, Goldhirsch A (1981) Medrossiprogesterone acetato (MAP) ad alto e basso dosaggio: uno studio randomizzato in pazienti con carcinoma avanzato della mammella. Tumori [Suppl A] 67:68
Cornette JC, Kirton KT, Duncan GW (1971) Measurement of medroxyprogesterone acetate (Provera) by radioimmunoassay. J Clin Endocrinol Metab 33:459–466
De Lena M, Brambilla C, Valagussa P, Bonadonna G (1979) MPA in metastatic breast cancer previously treated with chemotherapy. Cancer Chemother Pharmacol 2:175–180
Ganzina F (1979) High-dose medroxyprogesterone acetate in advanced breast cancer. A review. Tumori 65:563–585
Hesselius I, Johansson EDB (1981) Medroxyprogesterone acetate plasma levels after oral and i.m. administration in a long-term study. Acta Obstet Gynecol Scand 1018:65–70
Jeppson S, Johansson EDB (1976) MPA, estradiol, FSH and LH in peripheral blood after i.m. administration Depo-Provera to women. Contraception 14:461–469
Laatikainen T, Nieminen U, Adlercreutz H (1979) Plasma MPA levels following i.m. or oral administration in patients with endometrial carcinoma. Acta Obstet Gynecol Scand 58:95–99
Maskens AP, Hap B, Kozyreff VN, Callewaert W, Lion G, Van den Abbeele G (1980) Serum levels of medroxyprogesterone acetate under various treatment schedules. AACR Abstract 21:165
Mattson W (1978) High dose medroxyprogesterone acetate treatment in advanced mammary carcinoma. A phase II investigation. Acta Radiol Oncol 17:387
Ortiz A, Hiroi M, Stanczyk FZ, Goebelsmann U, Mishell DR (1977) Serum MPA concentrations and ovarian function following i.m. injection of Depo-MPA. J Clin Endocrinol Metab 44:32–38
Pannuti F, Martoni A, Lenaz GR, Piana E, Nanni P (1978) A possible new approach to the treatment of metastatic breast cancer: massive doses of medroxyprogesterone acetate. Cancer Treat Rep 62:499–504
Robustelli della Cuna G, Calciati A, Bernardo Strada MR, Bumma C, Campio L (1978) High-dose MPA treatment in metastatic carcinoma of the breast. A dose response evaluation. Tumori 64:143–150
Salimtschik M, Mouridsen HT, Loeber J, Johansson E (1980) Comparative pharmacokinetics of medroxyprogesterone acetate administered by oral and i.m. route. Cancer Chemother Pharmacol 4:267–269
Sall S, Di Saia P, Morrow CP, Mortel R, Prem K, Thigpen T, Creasman W (1979) A comparison of medroxyprogesterone serum concentrations by the oral or i.m. route in patients with persistent or recurrent endometrial carcinoma. Am J Obstet Gynecol 135:647–650
Shrimanker K, Saxena BN, Fotherby K (1978) A radioimmunoassay for serum medroxyprogesterone acetate. J Steroid Biochem 9:359–363
Wagner JG (1975) Dosage regimen calculations: In: Fundamental of clinical pharmacokinetics. Drug Intelligence Publications, Hamilton Ill, p 129–172
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tamassia, V., Battaglia, A., Ganzina, F. et al. Pharmacokinetic approach to the selection of dose schedules for medroxyprogesterone acetate in clinical oncology. Cancer Chemother. Pharmacol. 8, 151–156 (1982). https://doi.org/10.1007/BF00255475
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00255475