Summary
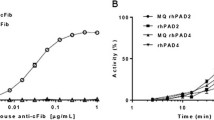
Synovial fluids of patients suffering from rheumatoid arthritis contain elevated levels of granulocyte (PMN) elastase in complex with alpha 1-proteinase inhibitor (alpha 1-PI), whereas free-elastase activity is usually not detectable. This absence of free enzymatic activity in joint effusions has cast some doubt on the pathophysiological relevance of PMN elastase in inflammatory joint destruction. Our in vitro experiments using bovine nasal cartilage demonstrate that incubation with elastase and alpha 1-PI in equimolar concentrations to or even in excess of the serum proteinase inhibitor resulted in significant tissue destruction as assessed by histological staining for proteoglycans, release of uronic acid from the matrix and loss of mechanical stability. Though in the supernatants containing alpha 1-PI, free-elastase activity was not detectable, immunofluorescent staining for elastase evidenced penetration of the enzyme into the matrix. Simultaneous measurements of the incubation media employing a sandwich enzyme-linked immunoadsorption assay (ELISA) revealed PMN elastase in complex with alpha 1-PI but without correlation to the parameters of tissue degradation. In comparison with the results obtained using the chromogenic substrate Suc-Ala-Ala-Ala-pNA (SAPA) for titration of alpha 1-PI against elastase, the employment of cartilage matrix showed that a fourfold increase in inhibitor concentration was necessary to achieve 100% enzyme inhibition. Hence, cartilage surface obviously interferes with the interaction between alpha 1-PI and elastase. Measurements of elastase-inhibitor concentrations or free enzymatic activity in synovial fluid seem to have limited value in predicting cartilage destruction.
Similar content being viewed by others
References
Barret AJ (1978) The possible role of neutrophil proteinases in damage to articular cartilage. Agents Actions 8:11–18
Gosh P (1978) Lysosomal enzymes in joint disease. Aust NZ J Med 8 (suppl 1):12–15
Hadler NM, Johnson AM, Spitznagel JK, Quinet RJ (1981) Protease inhibitors in inflammatory synovial effusions. Ann Rheum Dis 40:55–59
Dingle JT (1979) Recent studies on the control of joint damage: The contribution of the Strangeway Research Laboratory, Heberden Oration 1978. Ann Rheum Dis 38:201–214
Starkey PM, Barret AJ, Burleigh MC (1977) The degradation of articular collagen by neutrophil proteinases. Biochim Biophys Acta 483:386–397
Keiser H, Greenwald RA, Feinstein G, Janoff A (1976) Degradation of cartilage proteoglycan by human leukocyte granule neutral protease — A model of joint injury. J Clin Invest 57:625–632
Kleesiek K, Reinards R, Brackerts D, Neumann S, Lang H, Greiling H (1986) Granulocyte elastase as a new biochemical marker in the diagnosis of chronic joint diseases. Rheumatol Int 6:161–170
Virca GD, Mallya RK, Pepys MB, Schnebli HP (1984) Quantitation of human leukocyte elastase, cathepsin G, alpha 2-macroglobulin and alpha 1-proteinase inhibitor in osteoarthrosis and rheumatiod arthritis synovial fluids. Adv Exp Biol 167:345–353
Cohen G, Fehr K, Wagenhäuser FJ (1983) Leukocyte elastase and free collagenase activity in synovial effusions: relation to numbers of polymorphonuclear leukocytes. Rheumatol Int 3:89–95
Burkhardt H, Schwingel ML, Menninger H, Macartney HW, Tschesche H (1986) Oxygen radicals as effectors of cartilage destruction. Arthritis Rheum 29:379–387
Barthold PM, Page RC (1985) A microdetermination method for assaying glycosaminoglycans and proteoglycans. Anal Biochem 150:320–324
Stegemann H (1958) Mikrobestimmung von Hydroxyprolin mit Chloramin-T und p-Dimethylaminobenzaldehyd. Z Physiol Chem 311:41–45
Nakajima K, Powers JC, Castillo MJ, Ashe BM, Zimmermann M (1979) Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. J Biol Chem 245:4027–4031
Mowry RW (1956) Alcian blue techniques for the histochemical study of acidic carbohydrates. J Histochem Cytochem 4:407
Menninger H, Putzier R, Mohr W, Wessinghage D, Tillmann K (1980) Granulocyte elastase at the site of cartilage erosion by rheumatoid synovial tissue. Z Rheumatol 39:145–156
Menninger H, Burkhardt H, Röske W, Ehlebracht W, Hering B, Gurr E, Mohr W, Mierau HD (1981) Lysosomal elastase: Effect on mechanical and biochemical properties of normal cartilage, inhibition by polysulfonated glycosaminoglycan and binding to chondrocytes. Rheumatol Int 1:73–82
Engelbrecht S, Pieper E, Macartney HW, Rautenberg W, Wenzel HR, Tschesche H (1981) Separation of the human leukocyte enzymes alanine aminopeptidase, cathepsin G, collagenase, elastase and myeloperoxidase. Hoppe-Seyler's Z Physiol Chem 363:305–315
Tschesche H, Engelbrecht S, Wenzel HR (1979) In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 5. Verlag Chemie, Weinheim, pp 176–183
Maroudas A (1973) Physicochemical properties of articular cartilage. In: Freeman MAR (ed) Adult articular cartilage. Pitmann, London, pp 131–170
Janoff A (1973) Purification of human granulocyte elastase by affinity chromatography. Lab Invest 29:458–464
Stockley RA, Afford SC (1984) The effect of leukocyte elastase on the immunoelectrophoretic behavior of alpha 1-antitrypsin. Clin Sci 66:217–224
Hornebeck W, Soleilhac JM, Velebuy V, Robert L (1985) On the influence of the substrate (elastin) in elastase-alpha 1-antitrypsin interactions. Pathol Biol 33:281–285
Pritchard MH (1984) Synovial protease inhibitor ratios in erosive and nonerosive arthropaties. Ann Rheum Dis 43:50–55
Kimura H, Tateishi H, Ziff M (1977) Surface ultrastructure of rheumatoid articular cartilage. Arthritis Rheum 20:1085–1094
Taillard W, Lagier R (1969) Chondromalacia and arthritis of rheumatoid type. In: Hijmans W, Paul WD, Herschel H (eds) Early synovectomy in rheumatoid arthritis. Excerpta Medica Foundation, Amsterdam, pp 178–184
Drommer W (1982) Erreger bestimmen im Zusammenspiel mit Leukozyten und Fibrin die initiale Phase der Gelenkdestruktion. In: Otte P (Hrsg) Gelenkdestruktion bei Polyarthritis. Steinkopff, Darmstadt, pp 15–21
Velvart M, Fehr K, Baici A, Sommermeyer G, Knöpfel M, Cancer M, Salgam P, Böni A (1981) Degradation in vivo of articular cartilage in rheumatoid arthritis by leukocyte elastase from polymorphonuclear leukocytes. Rheumatol Int 1:121–130
Matheson NR, Wang PS, Travis J (1979) Enzymatic inactivation of human alpha 1-proteinase inhibitor by neutrophil myeloperoxidase. Biochim Biophys Res Comm 88:402–409
Johnson D, Travis J (1979) The oxidative inactivation of human alpha 1-proteinase inhibitor: further evidence for methionine at the reactive center. J Biol Chem 254:4022–4026
Carp H, Janoff A (1979) In vitro suppression of serum elastase inhibitory capacity by reactive oxygen species generated by phagocytosing polymorphonuclear leukocytes. J Clin Invest 63:793–797
Henson PM (1971) The immunologic release of constituents from neutrophil leukocytes. II. Mechanism of release during phagocytosis and adherence to nonphagocytosable surfaces. J Immunol 107:1547–1557
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burkhardt, H., Kasten, M., Rauls, S. et al. Interference of cartilage surface with interaction of granulocyte elastase with α1-proteinase inhibitor. Rheumatol Int 7, 133–138 (1987). https://doi.org/10.1007/BF00270466
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00270466