Summary
Studies were made on skeletal muscles obtained by biopsy and autopsy from a 59-year-old man with immunocyte dyscrasia and amyloidosis; he had progressive muscular stiffness with palpable tumors and ϰ-type Bence-Jones protein, but no overt myeloma.
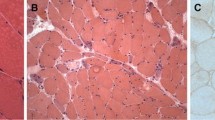
Histologically, much amyloid was seen deposited preferentially in the skeletal muscles, especially in the endo- and perimysia, forming tumors of various sizes and occasionally associating influx of amyloid into some muscle fibers, and in the smaller-sized blood vessel walls, frequently associated with extreme narrowing or occlusion of their lumina.
Most muscle fibers were replaced by much amyloid or showed extensive atrophy, having a characteristic circular space under the endomysium, which was concluded to be not due to compression atrophy but to ischemic atrophy. Ischemic neuropathy was observed and seemed to be responsible for the diffuse atrophy without spaces seen exceptionally in some parts. Amyloid was shown histochemically to contain AL as the major protein component, immunohistochemically to have a close relation with ϰ-type light chain, and fine-structurally to be formed just outside the basement membrane of the blood vessels and deposited in the endo- and perimysia through perivascular infiltration.
The clinicopathologic features of nine similar autopsy cases in the literature were compared with those in the present case.
The causes of damage in muscle fibers and of preferential deposition of amyloid in the skeletal muscles were discussed.
Similar content being viewed by others
References
Adams RD (1975) Amyloidosis. In: Adams RD (ed) Diseases of muscle. A study in pathology, 3rd edn. Harper and Row, Hagerstown New York Evanston San Franciso London, pp 461–463
Bornstein P, Sage H (1980) Structurally distinct collagen types. Ann Rev Biochem 49:957–1003
Cohen AS, Calkins E (1959) Electron-microscopic observation on a fibrous component in amyloid of diverse origins. Nature 183:1202–1203
Dahlin DC (1949) Amyloidosis. Proc Staff Meet Mayo Clin 24:637–648
Duance VC, Restall DJ, Beard H, Bourne FJ, Bailey AJ (1977) The location of three collagen types in skeletal muscle. FEBS Lett 79:248–252
Franklin EC, Calkins E (1978) Amyloidosis. In: Samter M (ed) Immunological diseases, vol 2, 3rd edn. Little, Brown and Company, Boston, pp 1158–1173
Glenner GG (1980) A retrospective and prospective overview of the investigations on amyloid and amyloidosis — The β-fibrillosis. In: Glenner GG, Costa PP, Freitas AF (eds) Amyloid and amyloidosis. Proceedings of the 3rd International Symposium on Amyloidosis, Póvoa de Vazim, Portugal, 1979, pp 3–13
Glenner GG, Ein D, Terry WD (1972) The immunoglobulin origin of amyloid. Am J Med 52:141–147
Hällen J, Rudin R (1966) Peri-collagen amyloidosis. A study of 51 cases. Acta Med Scand 179:483–499
Heller H, Missmahl HP, Sohar E, Gafni J (1964) Amyloidosis: its differentiation into peri-reticulin and peri-collagen types. J Pathol Bacteriol 88:15–34
Hirschl S (1969) Electron-microscopic analysis of human amyloid. J Ultrastruct Res 29:281–292
Hobbs JR (1973) An ABC of amyloid. Proc R Soc Med 66:705–710
Ikeda Y, Toyama K, Matsuki S, Asano S, Aiba M, Kano S (1973) Bence-Jones type multiple myeloma associated with amyloidosis and ileus: a case report. J Jpn Soc Int Med 62:274–279 (in Japanese)
Isobe T, Osserman EF (1974) Patterns of amyloïdosis and their association with plasma-cell dyscrasia, monoclonal immunoglobulins and Bence-Jones proteins. New Engl J Med 290:473–477
Kérnohan JW, Woltman HW (1942) Amyloid neuritis. Arch Neurol Psychiatry 47:132–140
Koletsky S, Stecher RM (1939) Primary systemic amyloidosis. Involvement of cardiac valves, joints and bones, with pathologic fracture of the femur. Arch Pathol 27:267–288
Lendrum AC, Slidders W, Fraser DS (1972) Renal hyalin — A study of amyloidosis and diabetic fibrinous vasculosis with new staining methods. J Clin Pathol 25:373–396
Linke RP (1982) Immunohistochemical identification and cross reactions of amyloid fibril proteins in senile heart and amyloid in familial polyneuropathy. Clin Neuropathol 1:172–182
Lubarsch O (1929) Zur Kenntnis ungewöhnlicher Amyloidablagerungen. Virchows Arch [Pathol Anat] 271:867–889
Martin JJ, Van Bogaert L, Van Damme J, Peremans J (1970) Sur une pseudo-myopathie ligneuse généralisée par amyloïdose primaire endomysio-vasculaire. J Neurol Sci 11:147–166
Miyazaki T, Chin I, Inoue S, Ozeki K, Koide T, Iio M (1973) An autopsy case of muscle amyloidosis. Saishin Igaku 28:801–809 (in Japanese)
Miyasaki K, Murao S, Tsunetoshi S, Koizumi N, Isobe T, Nakamura N, Nakano H, Ogino T, Hosokawa S (1979) Primary systemic amyloidosis. A case permitting pathological and biochemical investigations. Acta Pathol Jpn 29:157–169
Mokri B, Engel AG (1975) Duchenne dystrophy: Electronmicroscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fibers. Neurology 25:1111–1120
Osserman EF (1975) Plasma cell dyscrasias. In: Beeson PB, McDermott W (eds) Textbook of medicine. Saunders, Philadelphia London Toronto, pp 1535–1549
Osserman EF, Takatsuki K, Talal N (1964) The pathogenesis of “amyloidosis”. Studies on the role of abnormal gamma globulins and gamma globulin fragments of the Bence Jones (l-polypeptide) type in the pathogenesis of “primary” and “secondary amyloidosis” and the “amyloidosis” associated with plasma cell myeloma. Semin Hematol 1:3–86
Reimann HA, Koucky RF, Eklund CM (1935) Primary amyloidosis limited to tissue of mesodermal origin. Am J Pathol 11:977–988
Sheibner K, Meerbach W (1972) Skeletalmuskelveränderungen bei experimenteller Amyloidose der Maus. Zentralbl Allg Pathol 115:156–162
Shy GM (1966) Endomysial abnormalities. In: Minckler J (ed) Pathology of the nervous system, vol 1. McGraw-Hill, New York Toronto Sydney London, pp 756–757
Sternberger LA, Hardy PH, Jr, Cuculis JJ, Meyer HG (1970) Preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem 18:315–333
Terashima K, Matsuda A, Matsuda M, Yamagata H, Imai Y, Kojima M (1978) An autopsy case of skeletal muscle amyloidosis associated with ϰ-type IgA myeloma. J Jpn Soc RES 17:169–185 (in Japanese)
Uchino F (1967) Pathological study on amyloidosis. Role of reticuloendothelial cells in inducing amyloidosis. Acta Pathol Jpn 17:49–82
Walton J (1981) Amyloidosis. In: Walton J (ed) Disorders of voluntary muscle, 4th edn. Churchill Livingstone, Edinburg London Melbrourne New York, pp 226–227, 479
Wright JR, Calkins E, Humphrey RL (1977) Potassium permanganate reaction in amyloidosis. A histologic method to assist in differentiating forms of this disease. Lab Invest 36:274–281
Author information
Authors and Affiliations
Additional information
Supported by grant no. 81-01-09 from The National Center for Nervous, Mental and Muscular Disorders (NCMMD) of the Ministry of Health and Welfare of Japan
Rights and permissions
About this article
Cite this article
Ii, K., Hizawa, K., Nunomura, S. et al. Systemic amyloid myopathy — Light-microscopic and fine-structural study of the skeletal muscles with histochemical and immunohistochemical study of amyloid. Acta Neuropathol 64, 114–121 (1984). https://doi.org/10.1007/BF00695574
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00695574