Summary
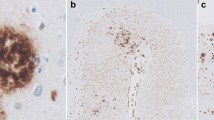
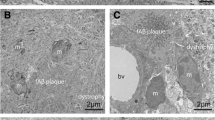
The spatial patterns of diffuse, primitive, classic (cored) and compact (burnt-out) subtypes of β/A4 deposits were studied in coronal sections of the frontal lobe and hippocampus, including the adjacent gyri, in nine cases of Alzheimer's disease (AD). If the more mature deposits were derived from the diffuse deposits then there should be a close association between their spatial patterns in a brain region. In the majority of tissues examined, all deposit subtypes occurred in clusters which varied in dimension from 200 to 6400 μm. In many tissues, the clusters appeared to be regularly spaced parallel to the pia or alveus. The mean dimension of the primitive deposit clusters was greater than those of the diffuse, classic and compact types. In about 60% of cortical tissues examined, the clusters of primitive and diffuse deposits were not in phase, i.e. they alternated along the cortical strip. Clusters of classic deposits appeared to be distributed independently of the diffuse deposit clusters. Cluster size of the primitive deposits was positively correlated with the density of the primitive deposits in a tissue but no such relationship could be detected for the diffuse deposits. This study suggested that there was a complex relationship between the clusters of the different subtypes of β/A4 deposits. If the diffuse deposits do give rise to the primitive and classic varieties then factors unrelated to the initial deposition of β/A4 in the form of diffuse plaques were important in the formation of the mature deposits.
Similar content being viewed by others
References
Allsop D, Haga SI, Haga C, Ikeda SI, Mann DMA, Ishii T (1989) Early senile plaques in Down's syndrome brains show a close relationship with cell bodies of neurons. Neuropathol Appl Neurobiol 15:531–542
Armstrong RA, Myers D (1992) β/A4 deposits and their relationship to senile plaques in Alzheimer's disease. Neuro Report 3:262–264
Armstrong RA, Myers D, Smith CUM (1989) Further studies on the patterns of senile plaques in senile dementia of the Alzheimer type (SDAT) with a hypothesis on the colonization of the cortex. Neurosci Res Commun 4:17–24
Armstrong RA, Myers D, Smith CUM (1990) The relationship between the spatial patterns of senile plaques and neurofibrillary tangles in Alzheimer's disease. Neurosci Res Commun 7:105–111
Armstrong RA, Myers D, Smith CUM Cairns N, Luthert PJ (1992) The spatial pattern of senile plaques, neurofibrillary tangles and A4 deposits in Alzheimer's disease. Neurosci Res Commun 10:27–33
Barrow CJ, Zagorski MG (1991) Solution structure of β peptide and it's constituent fragments: relation to amyloid deposition. Science 253:179–182
Beach TG, McGeer EG (1992) Senile plaques, amyloid β-protein and acetylcholinesterase fibers: laminar distributions in Alzheimer's disease striate cortex. Acta Neuropathol 83:292–299
Candy JM, Klinowski J, Perry RH, Perry EK, Fairbairn A, Oakley AE, Carpenter TA, Atack JR, Blessed G, Edwardson JA (1986) Aluminosilicates and senile plaque formation in Alzheimer's disease. Lancet 1:354–356
Delaère P, Duyckaerts C, He Y, Piette F, Hauw JJ (1991) Subtypes and differential laminar distributions of β/A4 deposits in Alzheimer's disease: relationship with the intellectual status of 26 cases. Acta Neuropathol 81:328–335
Edwards RJ, Clinton J, Gentleman SM, Roberts GW, Roystan MC (1992) Classification and quantification of plaque types in Alzheimer's disease using computerised image analysis. Neurodegeneration 1:65–71
Eikelenboom P, Rozemuller JM, Kraal G, Stain FC, McBride PA, Bruce ME, Fraser H (1991) Cerebral amyloid plaques in Alzheimer's disease but not in scrapie-affected mice are closely associated with a local inflammatory process. Virchows Arch [B] 60:329–336
Gallyas F (1971) Silver staining of Alzheimer's neurofibrillary changes by means of physical development. Acta Morphol Acad Sci Hung 19:1–8
Gardella JE, Ghiso J, Gorgone GA, Maratta D, Kaplan AP, Frangione B, Gorevic PD (1990) Intact Alzheimer's amyloid precursor protein (APP) is present in platelet membranes and is encoded by platelet mRNA. Biochem Biophys Res Commun 173:1292–1298
Giaccone G, Verga L, Finazzi M, Pollo B, Tagliavini F, Frangione B, Bugiani O (1990) Cerebral preamyloid deposits and congophilic angiopathy in aged dogs. Neurosci Lett 114:178–183
Hyman BT, Tanzi RE (1992) Amyloid, dementia and Alzheimer's disease. Curr Opin Neurol Neurosurg 5:88–93
Ikeda SI, Nobuo MD, Yanagisawa MB, Allsop D, Glenner GG (1990) Early senile plaques in Alzheimer's disease demonstrated by histochemistry, immunocytochemistry and electron microscopy. Hum Pathol 21:1221–1226
Marsland TA, Glees P, Erickson LB (1954) Modifications of the Glees silver impregnation for paraffin sections. J Neuropathol Exp Neurol 13:4245–4249
Myers D, Armstrong RA, Smith CUM (1988) The spatial arrangement patterns of senile plaques in senile dementia of the Alzheimer type (SDAT). Neurosci Res Commun 2:99–106
Ohgami T, Kitamoto T, Shin RW, Kaneko Y, Ogomori K, Takeishi J (1991) Increased senile plaques without microglia in Alzheimer's disease. Acta Neuropathol 81:242–247
Podlisny MB, Mammen AL, Schlossmacher MG, Palmert MR, Younken SG, Selkoe DJ (1990) Detection of soluble forms of the β-amyloid precursor protein in human plasma. Biochem Biophys Res Commun 167:1094–1101
Spargo E, Luthert PJ, Anderton BH, Bruce M, Smith D, Lantos PL (1990) Antibodies raised against different proteins of A4 protein identify a subset of plaques in Down's syndrome. Neurosci Lett 115:345–350
Verga L, Frangione B, Tagliavini F, Giaccone G, Migheli A, Bugiani O (1989) Alzheimer and Down's patients: cerebral preamyloid deposits differ ultrastructurally and histochemically from the amyloid of senile plaques. Neurosci Lett 105:294–299
Vinters HV, Pardridge WM (1986) The blood-brain barrier in Alzheimer's disease. Can J Neurol Sci 13:446–448
Wisniewski HM, Wegiel J (1991) Spatial relationships between astrocytes and classical plaque components. Neurobiol Aging 12:593–600
Yamaguchi H, Nakazato Y, Hirai S, Shoji M, Harigaya Y (1989) Electron micrograph of diffuse plaques: initial stage of senile plaque formation in the Alzheimer brain. Am J Pathol 135:593–597
Yamaguchi H, Nakazato Y, Shoji M, Takatama M, Hirai S (1991) Ultrastructure of diffuse plaques in senile dementia of the Alzheimer type: comparison with primitive plaques. Acta Neuropathol 82:13–20
Author information
Authors and Affiliations
Additional information
Supported in part by the Alzheimer's disease Society of the UK
Rights and permissions
About this article
Cite this article
Armstrong, R.A., Myers, D. & Smith, C.U.M. The spatial patterns of β/A4 deposit subtypes in Alzheimer's disease. Acta Neuropathol 86, 36–41 (1993). https://doi.org/10.1007/BF00454896
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00454896