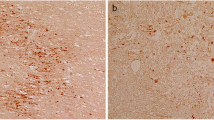
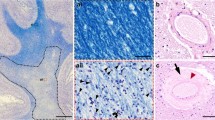
Abstract
Cerebral white matter lesions commonly observed in Binswanger's disease, multi-infarct encephalopathy and elderly people are neuropathologically characterized by diffuse incomplete demyelination and considered to be ischemic in nature. Arteriolosclerosis in the white matter is a common feature in these white matter lesions. To investigate a possible alteration of the distribution of amyloid precursor protein (APP), chromogranin A (CgA) and synaptophysin (Syn) in such white matter lesions, we examined 15 cases with white matter lesions and 5 without white matter lesions. Many bundles of axons with APP-like immunoreactivity (LI) were observed particularly in mild white matter lesions. Such bundles of axons showed similar but less intense CgA-LI and Syn-LI. They appeared to occur in areas with many ameboid or ramified microglia labeled with anti-leukocyte common antigen and few astrocytes labeled with anti-glial fibrillary acidic protein. In the center of moderate of severe white matter lesions bundles of axons with APP-LI were never observed. Since APP, CgA and Syn undergo fast axonal transport, and since following ischemic insults to central nervous system microglial reaction occurs earlier than astroglial changes, our results suggest that axonal damage, which induces disturbance of fast axonal transport, can occur even in the early stage of white matter lesions.
Similar content being viewed by others
References
Babikian V, Ropper AH (1987) Binswanger's disease: a review. Stroke 18: 2–12
Bööj S, Goldstein M, Fischer-Colbrie R, Dahlström A (1989) Calcitonin gene-related peptide and chromogranin A: presence and intra-axonal transport in lumbar motor neurons in the rat, a comparison with synaptic vesicle antigens in immunohistochemical studies. Neuroscience 30: 479–501
Cochran E, Bacci B, Chen Y, Patton A, Gambetti P, Autilio-Gambetti L (1991) Amyloid precursor protein and ubiquitin immunoreactivity in dystrophic axons is not unique to Alzheimer's disease. Am J Pathol 139: 485–489
Ferrer I, Bella R, Serrano MT, Marti E, Guionnet N (1990) Arteriolosclerotic leucoencephalopathy in the elderly and its relation to white matter lesions in Binswanger's disease, multi-infarct encephalopathy and Alzheimer's disease. J Neurol Sci 98: 37–50
Fisher CM (1989) Binswanger's encephalopathy: a review. J Neurol 236: 65–79
Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW (1993) β-Amyloid precursor protein (βAPP) as a marker for axonal injury after head injury. Neurosci Lett 160: 139–144
Glenner GG, Wong CW (1984) Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120: 885–890
Goto K, Ishii N, Fukasawa H (1981) Diffuse white-matter disease in the geriatric population: a clinical, neuropathological, and CT study. Radiology 141: 687–695
Griffin JW, Watson DF (1988) Axonal transport in neurological disease. Ann Neurol 23: 3–13
Hachinski VC, Potter P, Merskey H (1987) Leuko-araiosis. Arch Neurol 44: 21–23
Hattori H, Takeda M, Kudo T, Nishimura T, Hashimoto S (1992) Cumulative white matter changes in the gerbil brain under chronic cerebral hypoperfusion. Acta Neuropathol 84: 437–442
Kalaria RN, Bhatti SU, Palatinsky EA, Pennington DH, Shelton ER, Chan HW, Perry G, Lust WD (1993) Accumulation of the β amyloid precursor protein at sites of ischemic injury in rat brain. NeuroReport 4: 211–214
Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Müller-Hill B (1987) The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325: 733–736
Kawarabayashi T, Shoji M, Harigaya Y, Yamaguchi H, Hirai S (1991) Expression of APP in the early stage of brain damage. Brain Res 563: 334–338
Kitaguchi N, Takahashi Y, Tokushima Y, Shiojiri S, Ito H (1988) Novel precursor of Alzheimer's disease amyloid protein shows protease inhibitor activity. Nature 331: 530–532
Kitamoto T, Ogomori K, Tateishi J, Prusiner SB (1987) Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Invest 57: 210–226
Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL (1990) Precursor of amyloid protein in Alzheimer's disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA 87: 1561–1565
Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K (1985) Amyloid plaque core protein in Alzheimer's disease and Down syndrome. Proc Natl Acad Sci USA 82: 4245–4249
Morin PJ, Abraham CR, Amaratunga A, Johnson RJ, Huber G, Sandell JH, Fine RE (1993) Amyloid precursor protein is synthesized by retinal ganglion cells, rapidly transported to the optic nerve plasma membrane and nerve terminals, and metabolized. J Neurochem 61: 464–473
Morioka T, Kalehua AN, Streit WJ (1993) Characterization of microglia reaction after middle cerebral artery occlusion in rat brain. J Comp Neurol 327: 123–132
Nakamura Y, Takeda M, Niigawa H, Hariguchi S, Nishimura T (1992) Amyloid β-protein precursor deposition in rat hippocampus lesioned by ibotenic acid injection. Neurosci Lett 136: 95–98
Ohgami T, Kitamoto T, Tateishi J (1992) Alzheimer's amyloid precursor protein accumulates within axonal swellings in human brain lesions. Neurosci Lett 136: 75–78
Otsuka N, Tomonaga M, Ikeda K (1991) Rapid appearance of β-amyloid precursor protein immunoreactivity in damaged axons and reactive glial cells in rat brain following needle stab injury. Brain Res 568: 335–338
Scott JN, Parhard IM, Clark AW (1991) β-Amyloid precursor protein gene is differentially expressed in axotomized sensory and motor systems. Mol brain Res 10: 315–325
Shigematsu K, McGeer PL (1992) Accumulation of amyloid precursor protein in neurons after intraventricular injection of colchitine. Am J Pathol 140: 787–794
Shigematsu K, McGeer PL (1992) Accumulation of amyloid precursor protein in damaged neuronal processes and microglia following intracerebral administration of aluminum salts. Brain Res 593: 117–123
Shigematsu K, McGeer PL, Walker DG, Ishii T, McGeer EG (1992) Reactive microglia/macrophages phagocytose amylod precursor protein produced by neurons following neuronal damage. J Neurosci Res 31: 443–453
Shin R-W, Iwaki T, Kitamoto T, Tateishi J (1991) Hydrated autoclave treatment enhances TAU immunoreactivity in formalin-fixed normal and Alzheimer's disease brain tissues. Lab Invest 64: 693–702
Siman R, Card JP, Nelson RB, Davis LG (1989) Expression of β-amyloid precursor protein in reactive astrocytes following neuronal damage. Neuron 3: 275–285
Sisodia SS, Koo EH, Hoffman PN, Perry G, Price DL (1993) Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J Neurosci 13: 3136–3142
Stephenson DT, Rash K, Clemens JA (1992) Amyloid precursor protein accumulates in regions of neurodegeneration following focal cerebral ischemia in the rat. Brain Res 593: 128–135
Suenaga T, Hirano A, Llena JF, Ksiezak-Reding H, Yen S-H, Dickson DW (1990) Modified Bielschowsky and immunocytochemical studies on cerebellar plaques in Alzheimer's disease. J Neuropathol Exp Neurol 49: 31–40
Thiel G (1993) Synapsin I, synapsin II, and synaptophysin: marker proteins of synaptic vesicles. Brain Pathol 3: 87–95
Wakita H, Tomimoto H, Akiguchi I, Ohnishi K, Nakamura S, Kimura J (1992) Regional accumulation of amyloid β/A4 protein precursor in the gerbil brain following transient cerebral ischemia. Neurosci Lett 146: 135–138
Waxman SG, Black JA, Stys PK, Ransom BR (1992) Ultrastructural concomitants of anoxic injury and early post-anoxic recovery in rat optic nerve. Brain Res 574: 105–119
Weidemann A, König G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K (1989) Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell 57: 115–126
Winkler H, Fischer-Colbrie R (1992) The chromogranins A and B: the first 25 years and future perspectives. Neuroscience 49: 497–528
Wisniewski HM, Bancher C, Barcikowska M, Wen GY, Currie J (1989) Spectrum of morphological appearance of amyloid deposits in Alzheimer's disease. Acta Neuropathol 78: 337–347
Yamamoto K, Akai F, Yoshimine T, Yanagihara T (1987) Immunohistochemical investigation of cerebral ischemia after middle cerebral artery occlusion in gerbils. J Neurosurg 67: 414–420
Yamanouchi H, Sugiura S, Tomonaga M (1989) Decrease in nerve fibers in cerebral white matter in progressive subcortical vascular encephalopathy of Binswanger type: an electron microscopic study. J Neurol 236: 382–387
Author information
Authors and Affiliations
Additional information
Partly supported by a grant from the Ministry of Health and Welfare, Grant-in-Aid for Scientific Research on Priority Areas, and Scientific Research (C) (03670418) from the Ministry of Education, Science and Culture of Japan
Rights and permissions
About this article
Cite this article
Suenaga, T., Ohnishi, K., Nishimura, M. et al. Bundles of amyloid precursor protein-immunoreactive axons in human cerebrovascular white matter lesions. Acta Neuropathol 87, 450–455 (1994). https://doi.org/10.1007/BF00294171
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00294171