Abstract
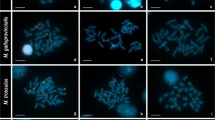
The major histone cluster (hisDNA) was mapped by fluorescent in situ hybridization (FISH) to mitotic chromosomes of Atlantic salmon, brown trout, and rainbow trout. The data reveal that in the three species hisDNA is tandemly repeated in a single locus. Southern blots of genomic DNA indicate that these clusters are representative of the vast majority of the histone genes in these species. Similar reiteration values were found among the three species. Genetic variability in the hisDNA was found only in brown trout for an EcoRI site.
Similar content being viewed by others
References
Allendorf FW, Thorgard GH (1984) Tetraploidy and the evolution of the salmonid fishes. In: Turner BJ (ed) Evolutionary genetics of fishes. Plenum, New York, pp 1–53
Connor W, Mezquita J, Winkfein J, States JC, Dixon GH (1984a) Organization of the histone genes in the rainbow trout (Salmo gairdneri). J Mol Evol 20:227–235
Connor W, States JC, Mezquita J, Dixon JC (1984b) organization and nucleotide sequence of rainbow trout histones H2A and H3 genes. J Mol Evol 20:236–250
Dalgleish R (1987) Gene cloning and analysis: a laboratory guide. Blackwell Scientific Publications
D'Andrea RJ, Coles LS, Lesnikowski C, Tabe L, Wells JRE (1985) Chromosomal organization of chicken histone genes: Preferred associations and inverted duplications. Mol Cell Biol 5:3108–3115
Delany ME, Bloom SE (1984) Replication banding patterns in the chromosomes of rainbow trout. J Hered 75:431–434
Englander E, Moav B (1989) Cloning and characterization of a histone gene family in Tilapia fish. Biochim Biophys Acta 1007:277–282
Fitch HA, Strausbaugh LD, Barrett V (1990) On the origin of tandemly repeated genes: Does histone gene copy number in Drosophila reflect chromosomal location? Chromosoma 99:118–124
Freije JP, Pendás AM, Velasco G, Roca A, Abrahamson M, López-Otín C (1993) Localization of the human cystatin D gene to chromosome 20p11.21 by in situ hybridization. Cytogenet Cell Genet 62:29–131
Gall JG, Stephenson CE, Erba HP, Diaz MO, Barsacchi-Pilone G (1981) Histone genes are located at the sphere loci of newt Lampbrush chromosomes. Chromosoma 84:159–171
Hammilton KE, Ferguson A, Taggart JB, Tomasson T, Walker A, Fahy E (1989) Postglacial colonisation of Brown trout (Salmo trutta): LDH-5 as a phylogeographic marker. J Fish Biol 35:651–664
Hankeln T, Schmidt ER (1991) The organization, localization and nucleotide sequence of the histone genes of the midge Chironomus thummi. Chromosoma 101:25–31
Hartley SE (1987) The chromosomes of salmonid fishes. Biol Rev 62:197–214
Hartley SE, Horne MT (1984) Chromosome polymorphism and constitutive heterochromatin in the Atlantic salmon Salmo salar. Chromosoma 89:377–380
Macleod AR, Wong NCW, Dixon GH (1977) The aminoacid sequence of trout testis histone H1. Eur J Biochem 78:281–291
Maxson R, Mohun T, Gormezano G, Childs G, Kedes L (1983a) Distinct organizations and patterns of expression of early and late histone gene sets in the sea urchin. Nature 301:120–125
Maxson M, Cohn R, Kedes L (1983b) Expression and organization of histone genes. Annu Rev Genet 17:239–277
May B, Stoneking M, Wright JE (1980) Joint segregation of biochemical loci in salmonidae. II. Linkage association from a hybridised Salvelinus genome (S. Namaycush x S. fontinalis). Genetics 95:707–726
Mayr B, Kalat M, Rab P (1988) Heterochromatin and band karyotypes of three species of salmonids. Theor Appl Genet 76:45–53
Megowan C, Davidson WS (1992) Unidirectional natural hybridisation between brown trout (Salmo trutta) and atlantic salmon (S. salar) in Newfoundland. Can J Fish Aquat Sci 49:1953–1958
Mezquita J, Connor W, Winfkein RJ, Dixon GH (1985) An H1 histone gene from rainbow Trout. J Mol Evol 21:209–219
Ohno S (1970) The enormous differences in genome sizes of fish as a reflection of natures's extensive experiments with gene duplication. Trans Am Fish Soc 99:120–130
Pendas AM, Morán P, Garcia-Vazquez E (1993a) Ribosomal RNA genes are interspersed throughout a heterochromatic chromosome arm in Atlantic salmon. Cytogenet Cell Genet 63:128–130
Pendas AM, Morán P, Garcia-Vazquez E (1993b) Multichromosomal location of ribosomal RNA genes and heterochromatin association in brown trout. Chromosome Res 1:63–67
Pendas AM, Morán P, Garcia-Vazquez (1993c) Replication pattern banding in Atlantic salmon. Genome 36:440–444
Pendas AM, Morán P, Garcia-Vazquez E (1993d) Improvements to Atlantic salmon anterior kidney metaphase yields following PHA injection. J Fish Biol 42:801–802
Philips RB, Hartley SE (1988) Fluorescent banding patterns of the chromosomes of the genus Salmo. Genome 30:193–197
Sanchez L, Martinez P, Viñas A, Bouza C (1990) Analysis of the structure and variability of nucleolar organizer regions of Salmo trutta by C-, Ag-, and restriction endonuclease banding. Cytogenet Cell Genet 54:6–9
Schmidtke J, Zences MT, Weiler C, Bross K, Engel W (1976) Gene action in fish of tetraploid origin. IV. Ribosomal DNA amount in Clupeoid and Salmonid fish. Biochem Genet 14:293–297
Sittman D, Chiu I, Pan C, Cohn R, Kedes L, Marzluff L (1981) Isolation of two clusters of mouse histone genes. Proc Natl Acad Sci USA 78:4078–4820
States JC, Connor W, Wosnick MA, Aiken JM, Gedamu L, Dixon GH (1982) Nucleotide sequence of a protamine component CII of gene of Salmo gairdnerii. Nucleic Acids Res 10:4551–4563
Taggart JB, Hynes RA, Prodöhl PA, Ferguson A (1992) A simplified protocol for routine total DNA isolation from salmonid fishes. J Fish Biol 40:963–965
Triputti P, Emanuel ES, Croce CM, Green LG, Stein GS, Stein JL (1986) Human histone genes map to multiple chromosomes. Proc Natl Acad Sci USa 83:3185–3188
Wiegant J, Ried T, Nederlof PM, van derPloeg M, Tanke HJ, Raap AK (1991) In situ hybridization with fluoresceinated DNA. Nucleic Acids Res 19:3237–3241
Winkfein RJ, Connor W, Mezquita J, Dixon G (1985) Histone H4 and H2B in rainbow trout (Salmo gairdnerii). J Mol Evol 22:1–19
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pendás, A.M., Morán, P. & García-Vázquez, E. Organization and chromosomal location of the major histone cluster in brown trout, Atlantic salmon and rainbow trout. Chromosoma 103, 147–152 (1994). https://doi.org/10.1007/BF00352324
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00352324