Summary
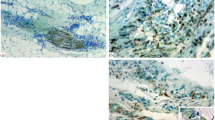
The presence and localization of fibronectin in normal and mechanically injured aorta in rabbits was studied using an indirect immunoperoxidase technique on tissue specimens fixed in formaldehyde, embedded in paraffin and pretreated with pepsin. The effect on staining quality of treatment with testicular hyaluronidase prior to immunoperoxidase staining was also examined. In the intima from normal aorta fibronectin was present in the subendothelial basal layer, along the internal and external elastic laminae, around and between the smooth muscle cells of the media and along the collagen and elastic fibres in the adventitia. Sixteen days after a single mechanical dilatation of the descending thoracic aorta all animals developed gross atherosclerotic-like changes. Microscopic examination revealed prominent neo-intimal hyperplasia with subendothelial, cushion-like thickenings but no medial or adventitial alterations. Fibronectin, in increased amounts, was found between and around the endothelial cells and in the subendothelial thickenings between the proliferating smooth muscle cells in relation to the fine, thin elastic and argyrophilic fibres. In the media and adventitia the amount and distribution of fibronectin was indistinguishable from uninjured control aortas. Treatment with testicular hyaluronidase before immunoperoxidase staining resulted in a higher staining resolution in normal and injured aorta. The conspicuous observation in the present study is that fibronectin exclusively accumulates in areas of tissue repair. The origins and functions of fibronectin during tissue injury and repair are discussed.
Similar content being viewed by others
References
Alami SY, Hampton JW, Race GJ, Speer RJ (1968) Fibrin stabilizing factor (Factor XIII). Am J Med 44:1–7
Borg TK, Gay RE, Johnson LD (1982) Changes in the distribution of fibronectin and collagen during development of the neonatal rat heart. Collagen Rel Res 2:211–218
Burke JM, Balian G, Ross R, Bornstein P (1977) Synthesis of type I and III procollagen and collagen by monkey aortic smooth muscle cells in vitro. Biochemistry 16:3243–3249
Clark RAF, Dvorak HF, Colvin RB (1981) Fibronectin in delayed-type hypersensitivity skin reactions: Association with vessel permeability and endothelial cell activation. J Immunol 126:787–793
Clark RAF, Quinn JH, Winn HJ, Lanigan JM, Dellepella P, Colvin RB (1982) Fibronectin is produced by blood vessels in response to injury. J Exp Med 156:646–651
Clausen PP, Thomsen P (1978) Demonstration of hepatitis B-surface antigen in liver biopsies. Acta Pathol Microbiol Scand [A] 86:383–388
Clemmensen I, Andersen RB (1982) Different molecular forms of fibronectin in rheumatoid synovial fluid. Arthritis Rheum 25:25–31
Clemmensen I, Hølund B, Johansen N, Andersen RB (1982) Demonstration of fibronectin in human articular cartilage by an indirect immunoperoxidase technique. Histochemistry 76:51–56
Dixon AJ, Burns J, Dunnill MS, McGee J (1980) Distribution of fibronectin in normal and diseased human kidneys. J Clin Pathol 33:1021–1028
Garbarsch C, Matthiessen ME, Helin P, Lorenzen I (1970) Spontaneous aortic arteriosclerosis in rabbits of the Danish country strain. Atherosclerosis 12:291–300
Garbarsch C (1973) II Enzyme histochemistry of rabbit thoracic aorta following a single mechanical dilatation injury. Acta Histochem 46:300–313
Gomori G (1950) Aldehyde fuchsin. A new stain for elastic tissue. Am J Clin Pathol 20:665–666
Gordon H, Sweets H (1936) A simple method for the silver impregnation of reticulum. Am J Pathol 12:545–551
Grinnell F, Billingham RE, Burgess L (1981) Distribution of fibronectin during wound healing in vivo. J Invest Dermatol 76:181–189
Harboe N, Ingild A (1973) Immunization, isolation of immunoglobulin, estimation of antibody titre. Scand J Immunol 2 [Suppl 1]:161–164
Helin P, Lorenzen I, Garbarsch C, Matthiessen ME (1971a) Repair in arterial tissue. Morphological and biochemical changes in rabbit aorta after a single dilatation injury. Circ Res 29:542–554
Helin P, Lorenzen I, Gabarsch C, Matthiessen ME (1971b) Arteriosclerosis in rabbit aorta induced by mechanical dilatation. Biochemical and morphological studies. Atherosclerosis 13:319–331
Hølund B, Clausen PP, Clemmensen I (1981) The influence of fixation and tissue preparation on the immunohistochemical demonstration of fibronectin in human tissue. Histochemistry 72:291–299
Hølund B, Clemmensen I, Junker P, Lyon H (1982) Fibronectin in experimental granulation tissue. Acta Pathol Microbiol Scand [A] 90:159–165
Hølund B, Clemmensen I (1982) The value of hyaluronidase treatment of different tissues before demonstration of fibronectin by the indirect immunoperoxidase technique. Histochemistry 76:517–525
Ishida T, Tanaka K (1982) Effects of fibrin and fibrinogen degradation products on the growth of rabbit aortic smooth muscle cells in culture. Atherosclerosis 44:161–174
Jaffe EA, Mosher DF (1978) Synthesis of fibronectin by cultured human endothelial cells. J Exp Med 147:1779–1791
Kurkinen M, Vaheri A, Roberts P, Stenman S (1980) Sequential appearance of fibronectin and collagen in experimental granulation tissue. Lab Invest 43:47–51
Lanser ME, Saba TM, Scovill WA (1980) Opsonic glycoprotein (plasma fibronectin) levels after burn injury. Ann Surg 192:776–782
Lendrum AC, Fraser DS, Slidders W, Henderson R (1962) Studies on the character and staining of fibrin. J Clin Pathol 15:401
Lillie RD (1965) Histopathologic technic and partial histochemistry, 3rd ed. McGraw-Hill, New York London
Linder E, Stenman S, Lehto V-P, Vaheri A (1978) Distribution of fibronectin in human tissues and relationship to other connective tissue components. Ann NY Acad Sci 312:151–159
Linder E, Miettinen A, Törnroth T (1980) Fibronectin as a marker for the glomerular mesangium in immunohistology of kidney biopsies. Lab Invest 42:70–75
Montes GS, Krisztán RM, Shigihara KM, Tokoro R, Mourao PAS, Junqueira LCU (1980) Histochemical and morphological characterization of reticular fibres. Histochemistry 65:131–141
Mosesson MW, Amrani DL (1980) The structure and biologic activities of plasma fibronectin. Blood 56:145–158
Mosher DF (1975) Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem 25:6614–6621
Natali PG, Galloway D, Nicotra MR, Demartino C (1981) Topographic association of fibronectin with elastic fibers in the arterial wall. An immunohistochemical study. Connect Tissue Res 8:199–204
Nowack H, Gay S, Wick G, Becker U, Timpl R (1976) Preparation and use in immunohistology of antibodies specific for type I and type III collagen and procollagen. J Immunol Methods 12:117–124
Oberley TC, Mosher DF, Mills MD (1979) Localization of fibronectin within the renal glomerulus and its production by cultured glomerular cells. Am J Pathol 96:651–662
Oh E, Pierschbacher M, Ruoslahti E (1981) Deposition of plasma fibronectin in tissues. Proc Natl Acad Sci USA 78:3218–3221
Remuzzi G, Marchesi E, de Gaetano G, Donati MB (1977) Abnormal tissue repair in Glanzmann's thrombasthenia. Lancet 1:374–375
Ruoslahti E, Engvall E, Hayman EG (1981) Fibronectin: Current concepts of its structure and functions. Cell Res 1:95–128
Stemerman MB, Ross R (1972) Experimental arteriosclerosis. I Fibrous plaque formation in primates, an electron microscope study. J Exp Med 136:769–789
Stenman S, Vaheri A (1978) Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med 147:1054–1064
Stenman S, von Smitten K, Vaheri A (1980) Fibronectin and atherosclerosis. Acta Med Scand [Suppl] 642:165–170
Wartiovaara J, Linder E, Ruoslahti E, Vaheri A (1974) Distribution of fibroblast surface antigen. Association with fibrillar structures of normal cells and loss upon viral transformation. J Exp Med 140:1522–1533
Yamada KM, Olden K (1978) Fibronectins — adhesive glycoproteins of cell surface and blood. Nature 275:179–184
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jensen, B.A., Hølund, B. & Clemmensen, I. Demonstration of fibronectin in normal and injured aorta by an indirect immunoperoxidase technique. Histochemistry 77, 395–403 (1983). https://doi.org/10.1007/BF00490900
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00490900